This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Assessment of mRNA SARS CoV-2 vaccine after administration of tixagevimab/cilgavimab, using B cell analysis
A new method to assess the status of immune responses to specific antigens in detail by analysis using the B cell receptor (BCR) repertoire has been developed by a research group, consisting of Assistant Professor Yohei Funakoshi, Associate Professor Kimikazu Yakushijin, Professor Hironobu Minami (Kobe University Graduate School of Medicine), Associate Professor Goh Ohji (Kobe University Hospital), and Researcher Takaji Matsutani (Repertoire Genesis Inc.).
These research results were published online in the British Journal of Haematology.
Previously, B-cell receptor (BCR) repertoire analysis has provided gene sequence data of antibodies (B cell receptors), but it was difficult to use this data to evaluate the immunological reactions to specific antigens.
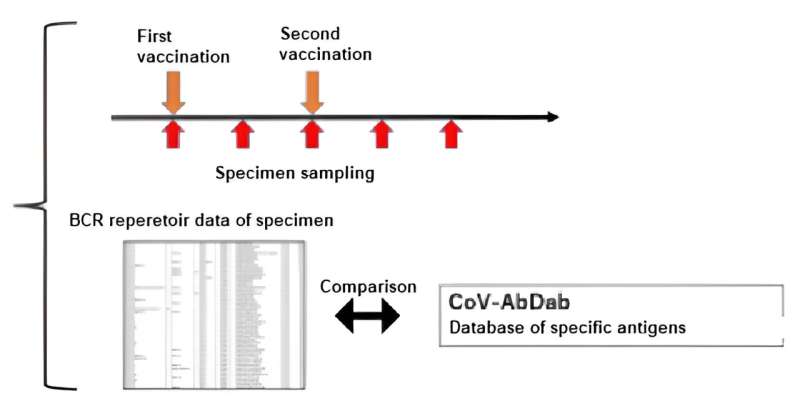
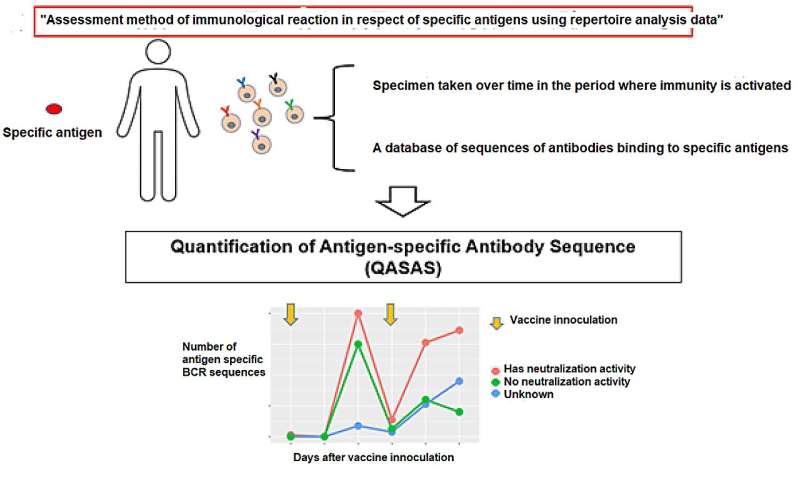
During the repertoire analysis, the team found that the immunological reactions could be evaluated by "sampling over time in the short time period wherein immunity is activated" and "utilizing the BCR gene sequence database for specific antigens."
This analysis is not the conventional analysis using protein level, such as "the measurement of antibody titers using the ELISA method," but an analysis using mRNA level. Therefore, evaluation is possible for samples with extremely elevated antibody titers after prophylactic administration of antibody preparations (proteins).
Detailed information can be derived, such as antibody epitopes expressed in immunological reactions or neutralization activity, if there is additional information in respect of the antibodies used. By utilizing this method to investigate immune responses following the administration of mRNA vaccines, it will be possible to evaluate the functionality of mRNA formulations.
In recent years, genetically-engineered antibody preparations, such as tixagevimab and cilgavimab, have been widely administered as preventative drugs, primarily for cases with compromised immune systems where there is insufficient antibody production after inoculation with mRNA SARS-CoV-2 vaccines.
However, say the researchers, antibody prophylaxis is not a complete substitute for vaccination. It is recommended that regular vaccines be administered even in cases where prophylaxis has already been administered. In such cases, the immunological reaction is typically assessed after vaccine inoculation by measuring antibodies using the ELISA method.
Due to the presence of significant amounts of antibodies from the prophylactic preparations, the reactions after vaccine inoculation cannot be accurately evaluated using the ELISA method. For this reason, there is a need for an assessment method to evaluate the immune response after vaccine inoculation, focusing on mRNA levels rather than protein levels.
The research group considers the BCR repertoire analysis, which analyzes the sequences of antibodies (BCR) using mRNA, is an effective method for mRNA level evaluation. However, there is no well-established assessment method for specific antigen reactions utilizing huge repertoire data analysis.
By conducting "sampling over time in the short time period wherein immunity is activated" and "availing of the BCR gene sequence database in respect of specific antigens" in implementing repertoire analysis, the researchers found that it was possible to assess the immunological reactions in respect of specific antigens at the mRNA level.
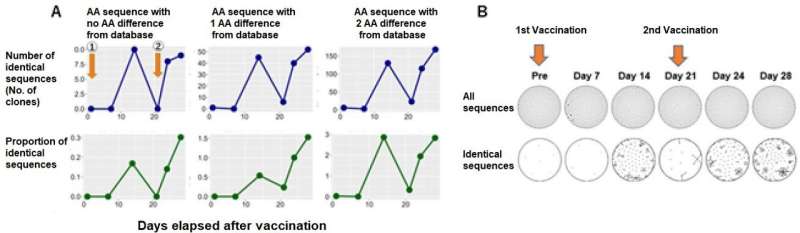
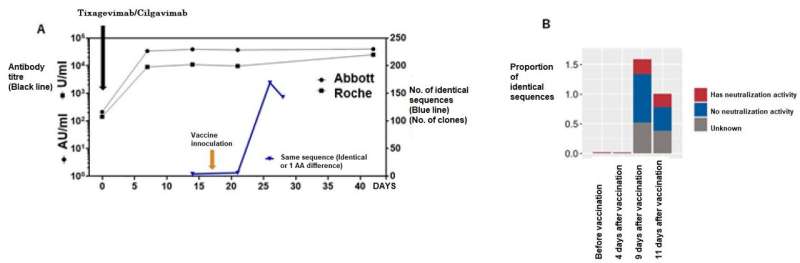
It is considered that the initial immunity is activated at about two weeks after inoculation with mRNA SARS CoV-2 vaccine. The researchers performed repertoire analysis at about one week after inoculation, when additional immunity is considered to be activated. On this occasion, the sequence information derived by repertoire analysis was extremely large, and it was difficult to assess the immunological reaction in respect of specific antigens using repertoire analysis alone.
For that reason, against the background of the recent COVID-19 pandemic, the researchers focused on the fact that a vast amount of information on SARS-CoV-2 antibody sequences has been accumulated and compiled into databases. Using this database, they focused on sequences that were reported to bind to a specific antigen (spike protein), or sequences similar to it, and conducted analysis, enabling them to evaluate post-vaccination responses.
Because this method analyzes post-vaccine response at the mRNA level, it can be used to evaluate post-vaccine response in immunocompromised patients who have received antibody prophylaxis.
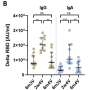
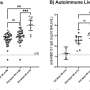
Patients who received recombinant antibodies (tixagevimab/silgavimab) after hematopoietic stem cell transplantation have shown extremely high antibody titers, as assessed by ELISA after administration of antibody drugs. Even with such a large amount of antibody protein in the body, the research group's method was able to clearly demonstrate a post-vaccination response.
Further developments
The research group termed the newly developed "Assessment method of immunological reaction in respect of specific antigens using repertoire analysis data (patent application filed)" as the "Quantification of antigen specific antibody sequence (QASAS)" method. The method can assess immune response even in situations where large amounts of antibodies are present in the body, e.g. due to administration of antibody drugs or infection/vaccination.
In addition, the advantages of this method don't stop there. If there is epitope or neutralization activity information in the database used, the assessment of the properties of the antibodies created from the immunologically reactive BCR sequence is enabled. The QASAS method has various advantages that the conventional ELISA method lacks, and it is considered suitable for assessing a wide range of mRNA formulations that are still under development.
More information: Yohei Funakoshi et al, Response to mRNA SARS‐CoV‐2 vaccination evaluated by B‐cell receptor repertoire after tixagevimab/cilgavimab administration, British Journal of Haematology (2023). DOI: 10.1111/bjh.18932