August 14, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Neanderthals, environment, and evolution behind SARS-CoV-2 immune responses
Research led by the Université Paris Cité, CNRS, France, has investigated factors driving variability in diverse immune responses to SARS-CoV-2.
In a paper, "Dissecting human population variation in single-cell responses to SARS-CoV-2," the team used single-cell RNA sequencing to analyze blood cells from diverse populations, comparing responses to SARS-CoV-2 against factors like genetics, age, sex, and comorbidities.
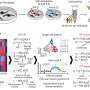
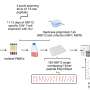
The study investigated immune responses to SARS-CoV-2 and the factors that could contribute to population differences in responses. With 222 healthy blood donors from different geographical regions and ancestries, researchers performed single-cell RNA sequencing on peripheral blood mononuclear cells to analyze their transcriptional responses to SARS-CoV-2 and influenza A virus exposure.
The findings reveal that SARS-CoV-2 induces weaker but more varied interferon-stimulated gene activity than the influenza A virus and has a unique pro-inflammatory signature in myeloid cells.
The findings, published in Nature, also illustrate that variability in population genetics, demographics and environment could explain why susceptibility to SARS-CoV-2 is not uniform.
Age
Older individuals generally exhibited weaker immune responses, which likely contributed to increased susceptibility and severity of COVID-19.
Comorbidities
Individuals with underlying health conditions showed altered immune responses, potentially leading to higher susceptibility and poorer outcomes.
Immune cell type composition
The study points out how proportions of memory cells in lymphoid lineages differ in individuals of African, European, and East Asian descent, potentially contributing to population disparities in cellular activation states.
For instance, African donors presented higher proportions of CD16+ monocytes and memory lymphocyte subsets, such as memory B cells and effector CD4+ T cells.
Environmental exposures
Past viral encounters, such as cytomegalovirus (CMV) infection, were seen to illicit differences in immune responses among different populations.
As an environmental factor, exposure to a virus can be a local or regional occurrence. Without knowledge of these exposures, immune response differences could be correlated to ancestry. The authors warn that this could lead to overestimating the effects of genetic ancestry on immune response variation.
For example, the team found that past CMV exposure accounts for up to 73% of the differences in immune cell proportions between African and European donors. These cell type compositions can impact transcriptional responses to SARS-CoV-2 but are environmental, not genetic in origin.
Genetic factors
Analysis revealed that genetics had a range of response effects on different cell types and immune responses. Common genetic variants can contribute to variations in immune response, but these effects are more pronounced in specific genes that show strong population differentiation.
An example is the rs1142888-G allele, which leads to higher expression of GBP7 in Europeans. This genetic difference likely arose due to positive selection in Europe thousands of years ago that could have been caused by past environmental exposure to pathogens.
Specific genetic variants that are associated with COVID-19 risk, in genes like IRF1, IFNAR2, and DR1, all influence type I interferon signaling, indicating the importance of efficient interferon signaling for favorable clinical outcomes.
Neanderthals
Past comingling and introgression of archaic human genes have contributed to present-day immune responses to SARS-CoV-2. Known Neanderthal genes have been retained in both European and East Asian populations that are specifically related to countering viral threats.
The researchers also identified previously unreported signals of Neanderthal introgression affecting immunity phenotypes. For example, a gene variant that decreases post-translational modification responses to SARS-CoV-2 and IA was found in 38% of Europeans and 22% of East Asians.
Another introgressed variant found in 43% of East Asians and less than 3% of Europeans downregulates a negative regulator of the cytosolic RNA-induced IFN response allowing more interferon release to counter the viral infection.
A Neanderthal haplotype reaching 61% frequency in East Asians and 24% in Europeans, tagged by rs9520848-C allele, is associated with higher basal expression of the cytokine gene TNFSF13B by a subset of T cells.
A Neanderthal genetic variant containing the rs2177336-T allele increases MUC20 expression in SARS-CoV-2-stimulated respiratory cells, particularly for CD4+ T cells. This was linked to decreased COVID-19 susceptibility in Eurasians.
The conserved nature of these archaic introgressions illustrates the deep history of pathogenic viruses testing human immune systems throughout evolution. While science and technology have created distinctly modern strategies to combat viral infections, there may be clues for applying that technology in the evolutionary and introgressively conserved genes.
Insights
The single-cell approach used in the study captured the complexity of immune responses across individuals and populations, revealing the interplay between environmental, genetic, and evolutionary factors.
Importantly the study unentangles the correlations between ancestry and recent environmental influences and illustrates how the environmental factors of the past influence inherited genetics.
With these factors elucidated, we can see how the immune system responds to an immediate novel threat and how it builds and maintains these responses over evolutionary time scales.
More information: Yann Aquino et al, Dissecting human population variation in single-cell responses to SARS-CoV-2, Nature (2023). DOI: 10.1038/s41586-023-06422-9
Insights into different populations' immune responses to SARS-CoV-2 infection, Nature (2023). DOI: 10.1038/d41586-023-02378-y
© 2023 Science X Network