This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Uncovering the role of KDM6A in epigenetic regulation of subtype plasticity in small cell lung cancer
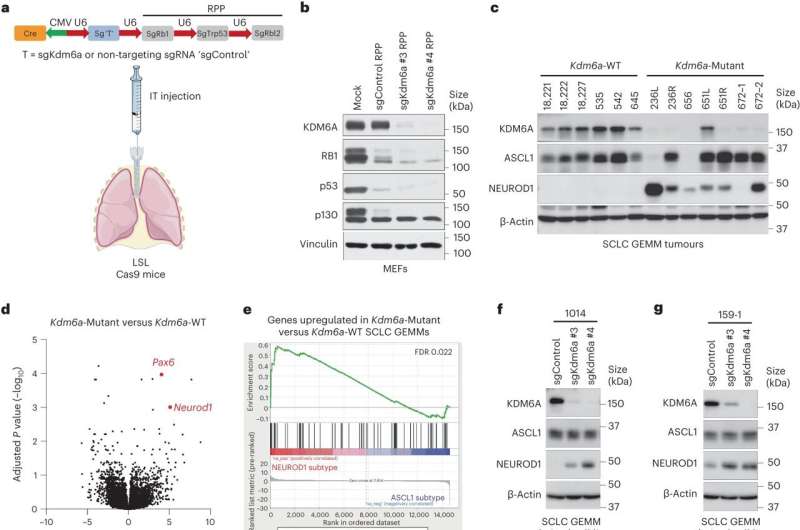
In a new study published in Nature Cell Biology, researchers used CRISPR/Cas9 in vivo somatic engineering to make mouse models of small cell lung cancer (SCLC) that either expressed KDM6A (KDM6A-WT) or were inactivated for KDM6A (KDM6A-Mutant). KDM6A is an epigenetic modifier that is mutated in human SCLC.
Researchers discovered a link between mutations in the KDM6A gene and the development of diverse subtypes within SCLCs. The loss or alteration of the KDM6A gene seems to play a role in promoting this diversity within the tumors.
Researchers found a mechanism involving KDM6A, which directly interacts with neuroendocrine genes. This interaction leads to the maintenance of a chromatin state that promotes the activation of these neuroendocrine genes, potentially playing a role in regulating processes related to the nervous and endocrine systems.
Mutations in epigenetic drivers like KDM6A can lead to plasticity in different subtypes of SCLC. A surprising observation is that a single mutation can have a profound impact on these subtypes.
Although KDM6A is likely not a good drug target directly, as its loss promotes intra-tumoral heterogeneity, this work suggests that identifying epigenetic drivers that when inhibited promote SCLC subtype plasticity toward more homogenous molecular subtypes may be effective strategies to combine with other therapeutic approaches that selectively target a subtype vulnerability.
Future studies will focus on identifying other druggable epigenetic drivers that may oppose the function of KDM6A or when inhibited block intra-tumoral subtype heterogeneity.
More information: Leslie Duplaquet et al, KDM6A epigenetically regulates subtype plasticity in small cell lung cancer, Nature Cell Biology (2023). DOI: 10.1038/s41556-023-01210-z