This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Global background rates study analyzes data from 197 million people for assessment of COVID-19 vaccine safety
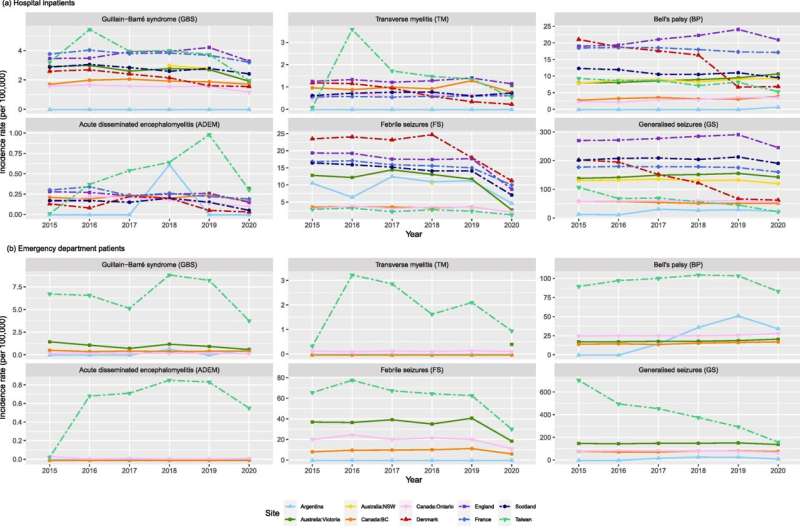
The U.S. CDC (Centers for Disease Control and Prevention) Global COVID Vaccine Safety Project has generated background incidence rates on a range of conditions designated as AESI (Adverse Events of Special Interest) for COVID-19 vaccine safety monitoring. Conditions studied included myocarditis, pulmonary embolism, and Guillain-Barré syndrome.
Eleven GVDN member sites implemented standardized methods and analyzed data from national or regional health care databases covering 197 million people from Europe, Asia, North and South America, and Oceania. The study captured data across five pre-pandemic years (2015–2019) and the initial year of the pandemic (2020).
Medical conditions can occur following vaccination but are not necessarily related to the use of the vaccine. Background rates can be used to compare how often the same medical conditions occur before and after a vaccine is introduced. This type of analysis can identify potential vaccine safety signals and support early safety investigations.
GVDN Co-Director Dr. Steven Black states, "With a collaborative global effort not yet seen in the vaccine safety space, the GVDN is pioneering these large-scale studies to assure the safety of vaccines and support public confidence in the safety of vaccines and guide policymaking decisions."
The findings were recently published in the journal Vaccine. Anastasia Phillips, lead author stated, "This study provides important data that can be used by many countries to investigate reports of medical events following vaccination and to support vaccine safety monitoring."
GVDN Co-Director Dr. Helen Petousis-Harris says, "The studies that we carry out will be made publicly available to support greater transparency, vaccine safety, more informed risk assessments, and stronger communication efforts." All data related to this background rates study can be viewed on a dashboard made available for public access at globalvaccinedatanetwork.org/Data-Dashboards.
More information: A. Phillips et al, Background rates of adverse events of special interest for COVID-19 vaccines: A multinational Global Vaccine Data Network (GVDN) analysis, Vaccine (2023). DOI: 10.1016/j.vaccine.2023.08.079