This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Phase I clinical trial shows treatment designed to clear senescent cells in Alzheimer's disease is safe
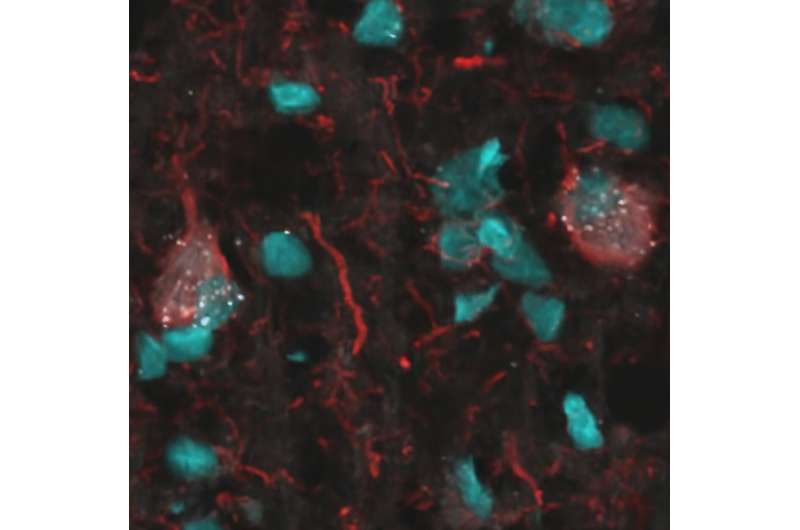
Alzheimer's disease is the most common cause of dementia that affects more than 6.5 million Americans, according to the Alzheimer's Association. To find effective treatments and slow the progression of this debilitating disease, researchers have made much progress in developing new drugs that target beta-amyloid plaques, one of the hallmarks of Alzheimer's disease.
Beta-amyloid plaques are accumulations of brain protein fragments, which can impact cognition. However, these recent drugs have only yielded modest results.
Now, scientists at Wake Forest University School of Medicine are reporting results from a Phase I trial in another area of promising research—cellular senescence.
The findings appear online today in Nature Medicine.
Senescent cells are old, sick cells that cannot properly repair themselves and don't die off when they should. Instead, they function abnormally and release substances that kill surrounding healthy cells and cause inflammation. Over time, they continue to build up in tissues throughout the body contributing to the aging process, neurocognitive decline and cancer.
"In 2018, we found evidence of senescent cells in human Alzheimer's disease," said Miranda Orr, Ph.D., associate professor of gerontology and geriatric medicine at Wake Forest University School of Medicine. "In mouse models, we also found that they contribute to brain cell loss, inflammation and memory impairment."
Researchers repurposed a U.S. Food and Drug Administration-approved drug designed to clear cancer cells (dasatinib) in combination with a flavonoid, a plant-derived antioxidant (quercetin).
"Our previous research has shown that the combination of these two drugs target senescent cells and allow them to die," Orr said. "We know that they cleared senescent brain cells in Alzheimer's disease mouse models, and they had already been shown to be safe in patients with other ailments."
For the current study, which was co-led by Mitzi Gonzales, Ph.D., of The University of Texas Health Science Center at San Antonio, the research team enrolled five participants aged 65 and older with symptoms of early-stage Alzheimer's disease. Participants received oral dasatinib plus quercetin over two consecutive days, followed by two weeks of no drugs. The cycle repeated six times for a total of 12 weeks.
"Our primary goal was to determine whether the medicines penetrated the central nervous system," Orr said. "We collected samples of patients' cerebrospinal fluid (CSF) before the first dose of medicine was given and after the last dose of medicine was given."
The research team also collected data on the safety and efficacy of the two drugs by monitoring side effects. They assessed biomarkers of senescence in CSF and blood, and also evaluated patients' cognition and brain images before treatment and after they completed the 12-week study.
They found that both dasatinib and quercetin levels increased in the blood, and dasatinib was detected in the CSF in four subjects. Quercetin was not detected in the CSF of any participants.
"We also determined that the treatment was safe, feasible and well-tolerated," Orr said. "There were no significant changes in brain function as determined by assessing memory and brain imaging to provide additional evidence that it is a safe therapy to evaluate further."
Researchers also saw evidence to suggest that the combination therapy cleared amyloid from the brain and lowered inflammation in the blood.
"However, we shouldn't over-interpret these results," Orr said. "There was a small number of people enrolled, there was no placebo arm to compare results."
Researchers also noted an increase in inflammation in CSF biomarkers. According to Orr, one possible explanation is a transient increase in inflammation when senescent cells are cleared. This increase could also be a marker of senescent cells dying or could potentially indicate inflammation associated with the treatment.
"We will need to monitor this closely in our next trial," said Orr, whose cellular senescence research is currently featured in a special issue of National Geographic focused on aging.
"Dr. Orr's research is a critical part of this pivotal moment in Alzheimer's research as the focus shifts from amyloid and tau, the classic disease hallmarks, toward how the biology of aging underlies the disease," said Howard Fillit, M.D., co-founder and chief science officer at the Alzheimer's Drug Discovery Foundation (ADDF).
"Aging is the leading risk factor for Alzheimer's, and it is important that the field explores new approaches for developing therapeutics, like senolytics, that target biological aging. Alzheimer's is a multifaceted disease, and similar to cancer, we will need multiple treatment options that can be combined and personalized to improve the outlook for millions of patients living with Alzheimer's."
Orr's research team is in the process of a larger $3 million, Phase II clinical trial to test the effects of clearing senescent cells with the combination therapy.
"We can confidently move forward with a larger study population and placebo arm knowing that the treatment is safe," Orr said. "We will also look forward to learning more about how the treatment may impact Alzheimer's disease biomarkers."
More information: Gonzales, M.M. et al, Senolytic therapy in mild Alzheimer's disease: a phase 1 feasibility trial, Nature Medicine (2023). DOI: 10.1038/s41591-023-02543-w. www.nature.com/articles/s41591-023-02543-w