This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Real-time live tissue sensitivity assay for pancreatic adenocarcinoma
A new research paper titled "Real time ex vivo chemosensitivity assay for pancreatic adenocarcinoma" has been published in Oncotarget.
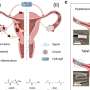
Patient-derived organoids (PDOs) and xenografts (PDXs) have been extensively studied for drug-screening. However, their usage is limited due to lengthy establishment time, high engraftment failure rates and different tumor microenvironment from original tumors. In this new study, researchers Dae Won Kim, Francisca Beato, Youngchul Kim, Alexandra F. Tassielli, Ruifan Dai, Jason W. Denbo, Pamela J. Hodul, Mokenge P. Malafa, and Jason B. Fleming from Moffitt Cancer Center developed real time-live tissue sensitivity assay (RT-LTSA) using fresh tumor samples to overcome these limitations.
"To overcome the major hurdles of the PDX-based assay, we developed real time LTSA (RT-LTSA) using fresh tumor samples. In this study, we report a reliable and reproducible RT-LTSA with resected fresh tumor samples to predict patients' clinical response to chemotherapy in pancreatic cancer," the researchers explain.
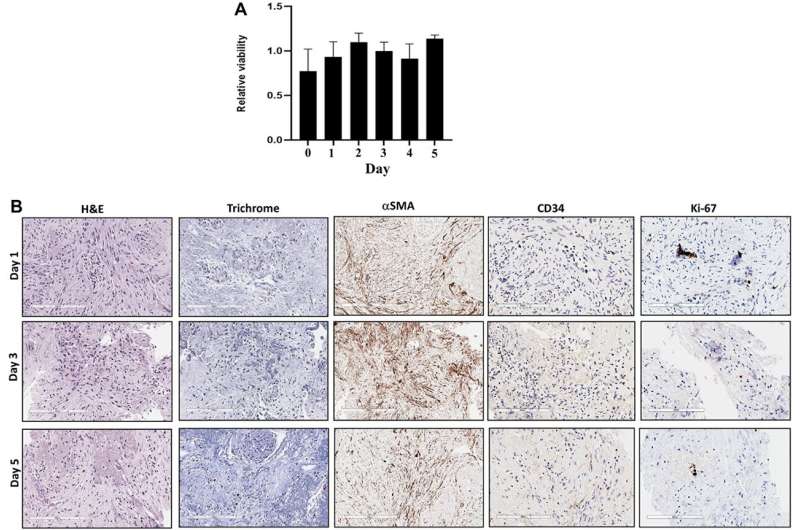
Tissue slices from resected pancreatic cancer samples were placed in 96-well plates, and the slices were treated with chemotherapeutic agents. The correlation between the chemosensitivity of tissue slices and each patient's clinical outcome was analyzed. The viability and tumor microenvironment of the tissue slices were well preserved over five days. The drug sensitivity assay results were available within five days after tissue collection.
While all four patients who received RT-LTSA sensitive adjuvant regimens did not develop recurrence, seven of eight patients who received resistant adjuvant regimens developed recurrence. The researchers observed significantly improved disease-free survival in the patients who received RT-LTSA sensitive adjuvant regimens (median: not reached versus 10.6 months, P = 0.02) compared with the patient who received resistant regimens. A significant negative correlation between RT-LTSA value and relapse-free survival was observed (Somer's D: −0.58; P = 0.016).
"RT-LTSA which maintains the tumor microenvironment and architecture as found in patients may reflect clinical outcome and could be used as a personalized strategy for pancreatic adenocarcinoma. Further, studies are warranted to verify the findings," the researchers conclude.
More information: Dae Won Kim et al, Real time ex vivo chemosensitivity assay for pancreatic adenocarcinoma, Oncotarget (2023). DOI: 10.18632/oncotarget.28508