This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Sterile barrier dressing increases efficiency and safety of UGPIV insertions, study finds
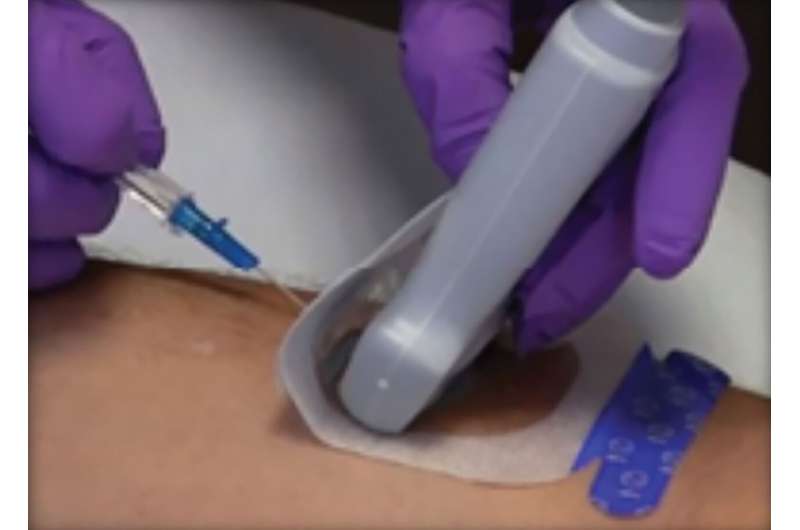
A unique sterile dressing can improve patient safety during ultrasound-guided peripheral IV (UGPIV) insertions by facilitating a standardized approach to aseptic technique, providing a barrier against contamination and ensuring effective securement and dressing adherence, according to a new pilot study by PICC Excellence and Sharp Healthcare (San Diego).
In this multi-center qualitative observational evaluation, individual clinician responses were collected immediately following 210 unique UGPIV procedures in which the sterile transparent barrier and securement dressing (UltraDrape Parker Laboratories) was used instead of a sterile probe sheath cover for probe protection.
Clinicians found the sterile barrier dressing improved ultrasound-guided insertions and was easy-to-use, with 98 percent of clinicians expressing a preference for UltraDrape over sterile probe covers. In addition, 99 percent of clinicians recommended adopting the new dressing to standardize UGPIV catheter insertions.
"UGPIV procedures are faster, safer, and less expensive with effective standardization," said study co-author and PICC Excellence CEO Nancy Moureau, RN, Ph.D., CRNI, CPUI, VA-BC. "Standardizing the procedure with UltraDrape will not only make the UGPIV procedure safer, but the improved efficiency and lower costs may also enable more patients to benefit from ultrasound-guided vascular access."
Nearly 60 percent of patients needing intravenous medication are considered to have difficult vascular access (DiVA) and require ultrasound guidance to achieve successful peripheral vascular access. It is estimated that there are 12 million UGPIV insertions performed annually in North America.
UltraDrape ensures aseptic technique with consistency following insertion policies, while maintaining sterility at the insertion site with its unique 4-layer (9-mm thick) design. Ultrasound gel is applied to a removable film layer on the back of the dressing, preventing the probe and gel from coming into direct contact with the insertion site.
Not only does this make for a cleaner insertion site and reduce the risk of contamination during insertion, but it also eliminates the post-insertion residual gel cleanup, preventing potential securement failure caused by inadequate gel removal.
Previous studies have revealed a tremendous amount of variability in how UGPIV procedures are performed. One survey of more than 26,000 clinicians involved in UGPIV insertions found clinically relevant inconsistencies in both supplies and aseptic techniques used for the procedure. That survey found providers used both sterile and non-sterile ultrasound gel, and that there was inconsistent use of probe protection, covers and transparent dressings.
The findings of this pilot study suggest the use of the sterile barrier and securement dressing may reduce the number of supplies needed, reducing the overall UGPIV procedure cost by as much as 67 percent per procedure. The cost of the procedure using UltraDrape and aseptic technique for the pilot was $4.63, compared to $10.38 for the standard practice using sheath probe covers and $13.54 for a full sterile insertion.
The faster and more efficient insertions result in a notable increase in patient comfort levels as well. "Patients often request a certified nurse to perform the procedures when an initial insertion attempt fails, trusting they are using safer and more successful techniques," Dr. Moureau explained.
The pilot study results were published in the International Journal of Nursing and Health Care Research and are available for no charge at the publisher's website.
More information: Michael Drafz et al, Multi-center Qualitative Observational Evaluation of Ultrasound Probe Protection using a Sterile Transparent Barrier and Securement Dressing to Standardize UGPIV Catheter Insertions, International Journal of Nursing and Health Care Research (2023). DOI: 10.29011/2688-9501.101418