This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Clinical trial finds live vaccinations safe for liver, kidney transplant recipients
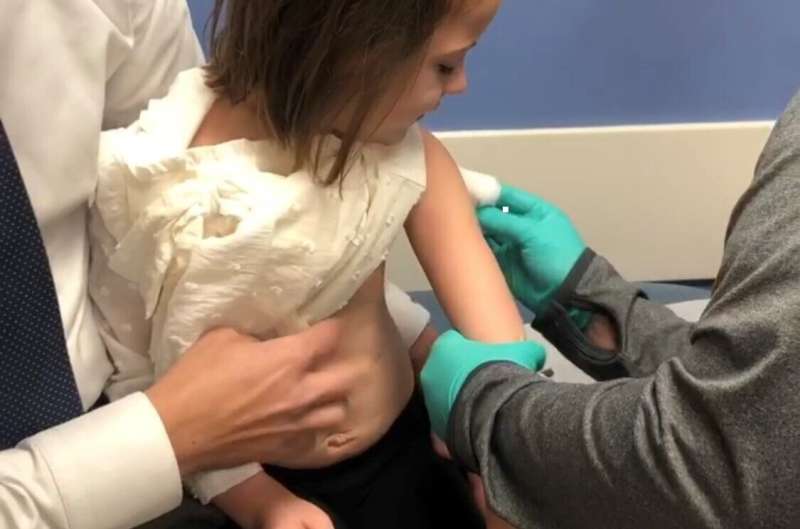
Live vaccinations provided to children who previously received liver or kidney transplants were found to be safe and prompted an immune response to guard against several life-threatening conditions, according to a new study published in JAMA Network Open.
The study, based on data from 18 organ transplant centers, was co-authored by Lara Danziger-Isakov, MD, MPH, interim director of the Division of Infectious Diseases at Cincinnati Children's, and Amy Feldman, MD, MSCS, medical director of the Liver Transplant Program at Children's Hospital Colorado.
The results are important because the rates of measles, mumps and the varicella-zoster virus that causes chicken pox are rising nationally and internationally, leaving immunocompromised children at risk for life-threatening conditions, according to Danziger-Isakov, senior author of the study.
"This shifts the paradigm of the approach to protecting this vulnerable population where live viral vaccines were previously avoided," Danziger-Isakov says. "This should enable children who have received organ transplants to integrate into their communities with more confidence and decreased risk for acquiring chicken pox or measles, which have both made a resurgence."
Global surges of measles and mumps were exacerbated by decreasing herd immunity as millions of vaccine doses were missed during the COVID-19 pandemic, leaving nonimmune organ transplant recipients at significant risk for community exposure, infection, disease and death from wild-type infection, says Feldman, first author of the study.
"Community acquisition of measles and varicella is a real risk for immunocompromised children in today's world," Feldman says. "These infections can be fatal in transplant recipients. Being able to administer live vaccines post-transplant, and provide immunity, is critical."
Historically, live vaccines to guard against measles, mumps, rubella (MMR) and varicella-zoster virus (VZV) have not been recommended after solid organ transplant due to concerns about a theoretical risk of vaccine strain infection in immunocompromised children. However, the authors reported no serious adverse events observed following the live vaccination of the children enrolled in the trial between Jan. 1, 2002, and Feb. 28, 2023.
Transplant recipients who participated in the trial received one to three doses of MMR vaccine and/or one to three doses of VZV vaccine. The cohort included 281 children on chronic immunosuppressive medications with a median age of 8.9 years at the time of the first post-transplant vaccine. The median time from transplant to enrollment in the study was 6.3 years. Safety data were collected after each vaccination, and antibody levels were measured at zero to three months and one year after vaccination.
The majority of children developed protective antibodies following vaccination—72% for varicella, 86% for measles, 83% for mumps and 99% for rubella. One year after vaccination, the majority of children who developed protective antibodies maintained that protection—77% for varicella, 92% for measles, 83% for mumps and 94% for rubella. Five children developed clinical varicella, but all of their conditions resolved within one week.
The findings suggest that live vaccinations may be safe and immunogenic after solid organ transplant in select pediatric recipients and can offer protection against circulating measles, mumps and varicella. Further research is needed to understand long-term maintenance of immunity after vaccination of children who receive organ transplants, as well as factors associated with immune response and clinical protection, the authors noted.
More information: Amy G. Feldman et al, Safety and Immunogenicity of Live Viral Vaccines in a Multicenter Cohort of Pediatric Transplant Recipients, JAMA Network Open (2023). DOI: 10.1001/jamanetworkopen.2023.37602