This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
An intravenous needle that irreversibly softens via body temperature on insertion
Intravenous (IV) injection is a common route for medical treatment worldwide as it induces rapid effects and allows continuous administration of medication by directly injecting drugs into the blood vessel. However, medical IV needles, made of hard materials such as stainless steel or plastic that do not mechanically match the soft biological tissues of the body, can cause critical problems in health care settings, starting from minor tissue damages in the injection sites to serious inflammation.
The structure and dexterity of rigid medical IV devices also enable the unethical reuse of needles for reduction of medical costs, leading to transmission of deadly blood-borne disease infections such as human immunodeficiency virus (HIV) and hepatitis B/C viruses. Furthermore, unintended needlestick injuries frequently occur in medical settings worldwide and are viable sources of infections, with IV needles as the most common medium of transmissible diseases.
For these reasons, the World Health Organization (WHO) in 2015 launched a policy on safe injection practices to encourage the development and use of "smart" syringes that have features to prevent re-use, after a tremendous increase in the number of deadly infectious disease worldwide due to medical sharps–related issues.
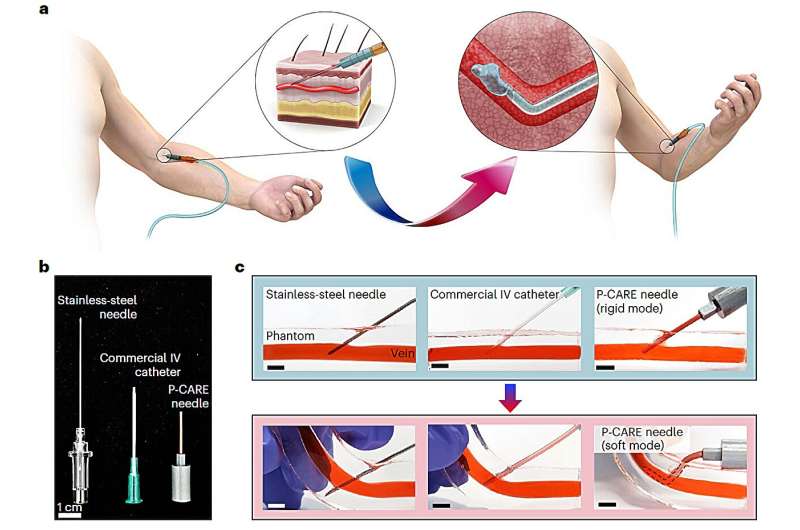
KAIST recently announced that Professor Jae-Woong Jeong and his research team from its School of Electrical Engineering, through convergent joint research with another team led by Professor Won-Il Jeong of the Graduate School of Medical Sciences, succeeded in developing the Phase-Convertible, Adapting and non-REusable (P-CARE) needle with variable stiffness that can improve patient health and ensure the safety of medical staff.
The new technology allows patients to move without pain at the injection site as it reduces the risk of damage to the wall of the blood vessel as patients receive IV medication. This is possible due to the needle's stiffness-tunable characteristics, which make it soft and flexible upon insertion into the body due to an increased temperature environment.
The needle adapts to the movement of thin-walled vein. It is also expected to prevent blood-borne disease infections caused by accidental needlestick injuries or unethical re-using of syringes as the deformed needle remains perpetually soft even after it is retracted from the injection site.
The results of this research, in which Karen-Christian Agno, a doctoral researcher of the School of Electrical Engineering at and Dr. Keungmo Yang of the Graduate School of Medical Sciences participated as co-first authors, was published in Nature Biomedical Engineering on October 30. The paper is titled, "A temperature-responsive intravenous needle that irreversibly softens on insertion."
"We've developed this special needle using advanced materials and micro/nano engineering techniques, and it can solve many global problems related to conventional medical needles used in health care worldwide," said Jae-Woong Jeong, Ph.D., an associate professor of Electrical Engineering at KAIST and a lead senior author of the study.
-
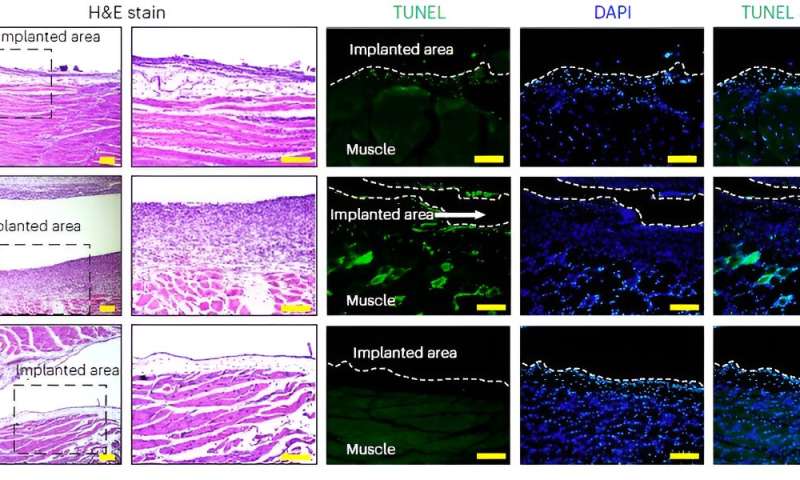
Biocompatibility test for P-CARE needle: Images of H&E stained histology (the area inside the dashed box on the left is provided in an expanded view in the right), TUNEL staining (green), DAPI staining of nuclei (blue) and co-staining (TUNEL and DAPI) of muscle tissue from different organs. Credit: The Korea Advanced Institute of Science and Technology (KAIST) -
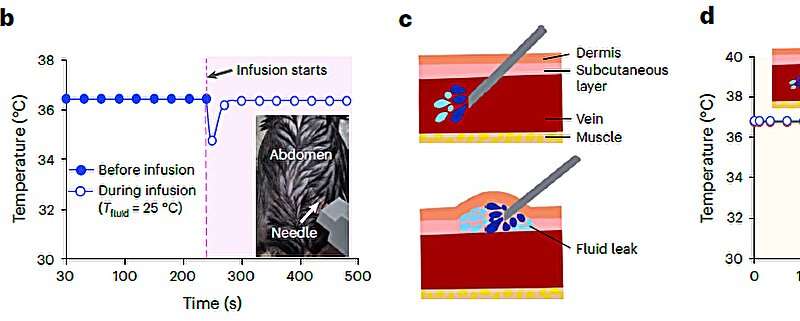
Conceptual images of potential utilization for temperature monitoring function of P-CARE needle integrated with a temperature sensor. (a) Schematic diagram of injecting a drug through intravenous injection into the abdomen of a laboratory mouse (b) Change of body temperature upon injection of drug (c) Conceptual illustration of normal intravenous drug injection (top) and fluid leakage (bottom) (d) Comparison of body temperature during normal drug injection and fluid leakage: when the fluid leak occur due to incorrect insertion, a sudden drop of temperature is detected. Credit: The Korea Advanced Institute of Science and Technology (KAIST)
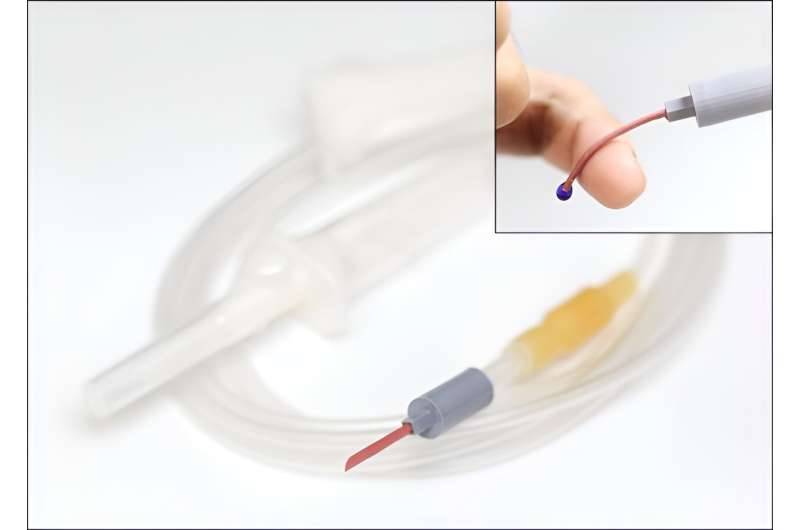
The softening IV needle created by the research team is made up of liquid metal gallium that forms the hollow, mechanical needle frame encapsulated within an ultra-soft silicone material. In its solid state, gallium has sufficient hardness that enables puncturing of soft biological tissues.
However, gallium melts when it is exposed to body temperature upon insertion, and changes it into a soft state like the surrounding tissue, enabling stable delivery of the drug without damaging blood vessels. Once used, a needle remains soft even at room temperature due to the supercooling phenomenon of gallium, fundamentally preventing needlestick accidents and reuse problems.
Biocompatibility of the softening IV needle was validated through in vivo studies in mice. The studies showed that implanted needles caused significantly less inflammation relative to the standard IV access devices of similar size made of metal needles or plastic catheters. The study also confirmed the new needle was able to deliver medications as reliably as commercial injection needles.
Researchers also demonstrated the possibility of integrating a customized ultra-thin temperature sensor with the softening IV needle to measure the on-site temperature, which can further enhance a patient's well-being. The single assembly of sensor-needle device can be used to monitor the core body temperature, or even detect if there is a fluid leakage on-site during indwelling use, eliminating the need for additional medical tools or procedures to provide the patients with better health care services.
The researchers believe that this transformative IV needle can open new opportunities for a wide range of applications particularly in clinical setups, in terms of redesigning other medical needles and sharp medical tools to reduce muscle tissue injury during indwelling use. The softening IV needle may become even more valuable as there is an estimated 16 billion medical injections administered annually on a global scale, yet not all needles are disposed of properly, according to a 2018 WHO report.
More information: Karen-Christian Agno et al, A temperature-responsive intravenous needle that irreversibly softens on insertion, Nature Biomedical Engineering (2023). DOI: 10.1038/s41551-023-01116-z