This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Cryoprotectants for red blood cells: Evaluating safety and effectiveness by in vitro measures
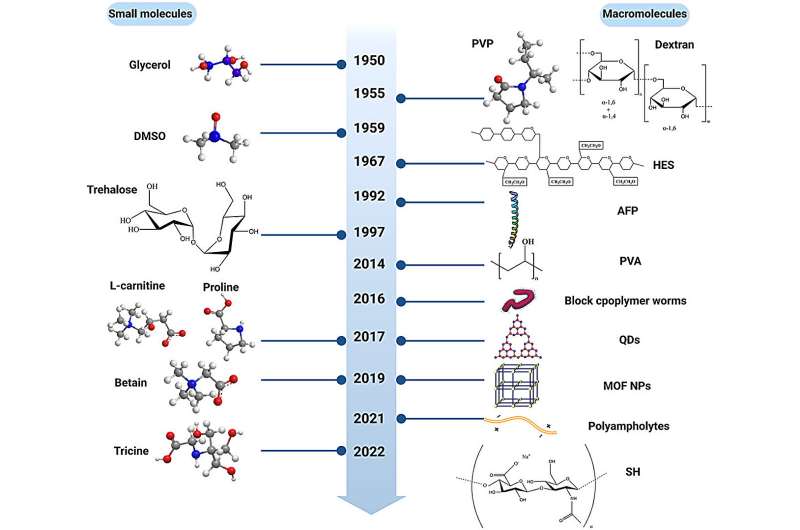
The cryopreservation of red blood cells (RBCs) holds great potential for ensuring timely blood transfusions and maintaining an adequate RBC inventory. Cryoprotectants (CPAs) are a series of biomaterials developed to reduce cryoinjuries. To achieve the high efficiency of RBC cryopreservation, suitable CPAs should be added before freezing.
Glycerol is the current state-of-the-art CPA for RBCs. Although it is often regarded as having low toxicity, the working concentrations during cryopreservation are up to 20% (in Europe) and 40% (in the U.S.), which causes severe side effects to RBCs. Many CPAs have been explored successfully as substitutes for glycerol, but there is still no uniform standard to evaluate their performance.
Some early studies only focused on RBC recovery, which is insufficient and inaccurate. Although some researchers have added functional indicators of thawed RBCs to evaluate CPAs, it is still not enough to fully determine their performance and may be an obstacle to the cryopreservation of RBCs and transfusion therapy.
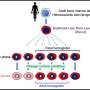
Recently, Hu Yuying and colleagues from Central South University summarized for the first time comprehensive methods to evaluate the performance of CPAs by ensuring their safety and effectiveness. The safety of CPAs can be assessed through their biocompatibility with RBCs. Most organic solvent CPAs show poor biocompatibility, which can lead to severe side effects. Therefore, it is necessary to evaluate the biocompatibility of CPAs, which can be determined by the hemolysis test and RBC morphology observation. The paper is published in the journal MedComm—Future Medicine.
The effectiveness is demonstrated by the properties of thawed RBCs, including membrane properties, protein activities, rheological properties, and metabolites levels. Osmotic fragility, morphology, membrane lipid peroxidation, membrane stiffness, PS exposure, and CD47 expression are employed as indicators of RBC membrane properties. Protein activities of RBCs are elucidated by different aspects: Hb leakage, Band-3 phosphorylation, ATPase activity, protein oxidation, and antioxidant enzyme activity. The rheological properties of RBCs include aggregation and deformability. ATP, 2,3-DPG, and NO are several important metabolites of RBCs. It must be noted that all studies have only been done at the laboratory level, and more experiments are needed for the clinical applications of thawed RBCs.
However, it is unrealistic to measure all the in vitro tests mentioned for the evaluation of CPAs. The important parameters include the biocompatibility test of CPAs and post-freeze-thaw/wash cell recovery used for evaluating the safety and effectiveness of CPAs, respectively, which are essential. In contrast, thawed cell functional properties, including membrane properties, protein activities, rheological properties, and metabolites levels, would just be nice to know.
To further ensure CPA effectiveness in cryopreserving RBCs for clinical applications, more experiments are needed before transfusion. Currently, the RBCs cryopreserved in CPAs are from sheep or avian, which cannot simply be extrapolated to human RBCs. Apart from the RBC species, a volume of at least 150 mL of packed RBCs for transfusion is needed, while most CPAs are tested on very small volumes of RBCs. Therefore, further studies are required to prove the safety and effectiveness of CPAs on a large volume of human RBCs.
Apart from the species and volume of RBCs, serologic compatibility between the recipient and the donor should be tested before transfusion. Pretransfusion testing of patients receiving allogeneic blood includes ABO and rhesus D (RhD) typing and antibody screens to detect unexpected antibodies to RBC antigens.
In conclusion, this paper reviewed the recent advances in CPAs for RBC cryopreservation and summarized the methods for determining the safety and effectiveness of CPAs comprehensively. More importantly, RBC cryopreservation can be used as a simple model for novel CPA screening, and research results from in vitro measures can initially ensure the biocompatibility and effectiveness of CPAs before progressing to nucleated cell cryopreservation. Therefore, the review can provide a new perspective on cryopreservation and promote the future development of biomedical research.
More information: Yuying Hu et al, Cryoprotectants for red blood cells: Evaluate safety and effectiveness by in vitro measures, MedComm—Future Medicine (2023). DOI: 10.1002/mef2.67