December 27, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Gut microbes may determine patients' response to a drug that delays onset of type 1 diabetes
The microbiome offers a motherlode of data about health and disease, and new findings suggest that antibodies to gut microbes can determine how well patients respond to a new monoclonal antibody drug that delays the onset of type 1 diabetes.
Increasingly, scientists are finding that the gut microbiome has unexpected relationships with health and disease. Research into the gut-brain axis, for example, has unveiled a surprising relationship between gut microbes and mental health. But medical investigators say the list is longer and the link to gut microbes equally complex.
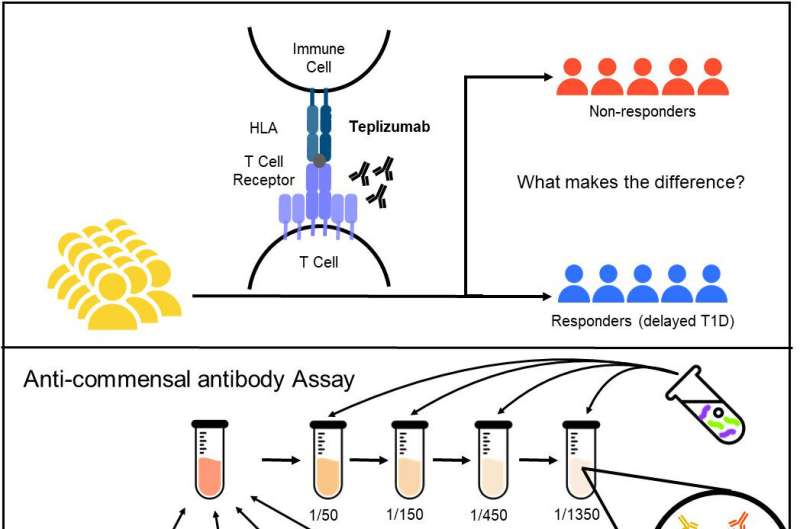
Now, clinical trial data have allowed researchers to track how the gut microbiome can influence patients' response to tepluzimab, a medication that delays type 1 diabetes. The monoclonal antibody therapy targets T cells and prevents them from destroying insulin-producing beta cells. The antibody is the first treatment approved by the U.S. Food and Drug Administration to postpone the metabolic disorder in high-risk individuals.
The FDA approved the drug based on results from a randomized clinical trial known as TrialNet-10 study, or the TN-10 study for short. Medical investigators from the University of Toronto revisited the TN-10 trial, studying more than 200 blood samples from 63 participants before and after teplizumab treatment.
Findings from the Toronto analysis, reported in the journal Science Translational Medicine, casts a new spotlight on the immune system's relationship with the microbiome, revealing how gut microbes can shape the progression of type 1 diabetes. With this new knowledge in hand, clinicians may better pinpoint patients who are most likely to respond to teplizumab.
Once known as juvenile diabetes because the disorder most frequently begins in childhood, the condition is linked to a constellation of potential causes. The disorder is tied to a turncoat immune system, which destroys insulin-producing beta cells in the pancreatic islets of Langerhans. Destruction of beta cells leads to lifelong insulin dependence.
Doctors say there are two other possible causes of type 1 diabetes: a genetic predisposition to the disease, and exposure to certain viruses. Either way—faulty DNA or viral exposure—the end result is a T cell attack on beta cells in the pancreas. Type 1 diabetes is categorized as an autoimmune disease, but is more precisely defined as an autoinflammatory condition.
"Immune-targeted therapies have efficacy for treatment of autoinflammatory diseases," writes Quin Yuhui Xie, lead author of a new investigation published in Science Translational Medicine. "For example, treatment with the T cell–specific anti-CD3 antibody teplizumab delayed disease onset in participants at high risk for type 1 diabetes in the TrialNet 10 trial.
"However, heterogeneity in therapeutic responses in TrialNet-10 and other immunotherapy trials identifies gaps in understanding disease progression and treatment responses," added Xie, a researcher in the Department of Medical Biophysics at the University of Toronto in Canada.
The FDA approved tepluzimab in November of 2022 amid findings that revealed not all patients in the TN-10 study experienced the same benefits. The reason for that discrepancy, Xie now says, may be explained by specific commensal bacteria. Commensal bacteria are so-called "friendly bacteria." They make up the microbiota, a diverse community numbering in the trillions inhabiting mucosal and epidermal surfaces in humans. These bacteria play critical roles in defense against pathogens and apparently in response to the drug teplizumab.
"We investigated anti-commensal antibody responses against a panel of taxonomically diverse intestinal bacteria species in sera from TN-10 participants before and after teplizumab or placebo treatment," Xie wrote.
The Toronto team theorized that differences in patients' responses might be explained by anti-commensal antibodies directed against commensal microbes in the gut microbiome. The team then analyzed antibody profiles in 228 serum samples from 63 participants in the TN-10 trial before and after teplizumab treatment.
Patients who had longer-lived antibody responses to three species of gut bacteria—Bifidobacterium longum, Enterococcus faecalis, and Dialister invisus—had more time on teplizumab treatment before being diagnosed as having type 1 diabetes. Clinical trial data revealed that patients with stronger immune responses against the three gut microbes tended to gain the most benefit from the drug's disease-delaying effects.
"The intestinal microbiome is a potential source of biomarkers," Xie concluded, noting the Toronto team "previously reported that antibody responses to gut commensal bacteria were associated with type 1 diabetes diagnosis, suggesting that certain antimicrobial immune responses may help predict disease onset."
More information: Quin Yuhui Xie et al, Immune responses to gut bacteria associated with time to diagnosis and clinical response to T cell–directed therapy for type 1 diabetes prevention, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.adh0353
© 2023 Science X Network