This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Knowing how clinicians make real-world decisions about drug-drug interactions can improve patient safety
Drug-drug interactions causing adverse effects are common and can cause significant patient harm and even death. A new study is one of the first to examine how clinicians become aware of, and process information about potential interactions and subsequently make their real-world decisions about prescribing. Based on these findings, the research team makes specific recommendations to aid clinician decision-making to improve patient safety.
"Drug-drug interactions are very common, more common than a lot of people outside the health care system expect. In the U.S., these interactions lead to hundreds of thousands of hospitalizations in any given year at an enormous cost," said study senior author Michael Weiner, M.D., MPH., of U.S. Department of Veterans Affairs, Regenstrief Institute and Indiana University School of Medicine. "Most of these drug interactions are preventable."
"This study was needed because we previously didn't understand how clinicians actually make decisions in assessing these interactions. No one had really taken apart the thinking process step-by-step to understand it from the beginning to the end. There's a patient; there's a drug and another drug."
"There is now a potential interaction. There's been a decision about how to resolve it following an assessment and then a resolution process. Understanding all this is very important if we are hoping to design improvements to the medical system that enhance patient safety."
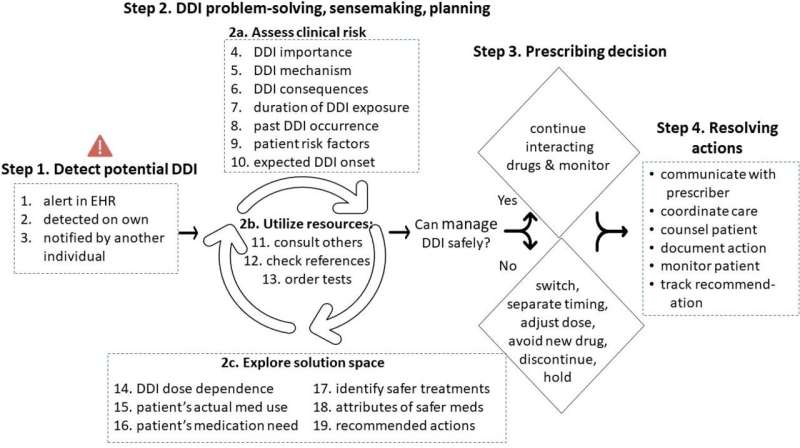
The research team focused on positive cases, where clinicians identified a drug-drug interaction concern and took action to help protect the patient. They analyzed all aspects of clinicians' decision-making process, especially specific cues they used to assess patients' clinical risk and identify safer treatment options.
Clinicians become aware of drug-drug interactions in different ways. In addition to their own knowledge and consultations with colleagues, reference books, or professional websites, the electronic health record (EHR) is a very common source of drug interaction alerts because all medications would ideally be logged, ordered, or tracked.
However, if a patient is prescribed drugs in multiple health systems, there typically is no integration of their EHR records. Reconciling all their medication information may be a formidable task for physicians, nurse practitioners, pharmacists, or other clinicians, who often operate under rigorous time constraints.
The study identified 19 cognitive cues clinicians rely on to detect and make decisions about drug-drug interactions. These cues include:
- information that influenced the interpretation of potential severity of drug-drug interaction
- type or degree of side effects or harms
- patient's expected duration of exposure to interaction
- patient-specific conditions that may increase risk of interaction
- patient's medical need for the medications
- characteristics of safer medications
Drug-drug interactions can be addressed by investigating alternative treatments that might be better or safer, altering dosage, as well as stopping or not prescribing a specific medication. Companion activities include educating patients about the warning signs of drug-drug interactions and related adverse events.
There may be situations where the risk of the interaction is considered acceptable based on the benefits and risks of the drugs being considered. But in other cases, a preventive strategy can involve either the patient's clinicians or the patient or both.
With the greater understanding of clinicians' cognitive processes related to drug-drug interactions in hospital or outpatient settings presented in this study, there is the potential to design and implement EHR system alerts that provide better, more actionable and more timely information to inform clinicians' decision-making process and ultimately to improve patient safety.
"This was a rewarding study, not only because of its important scientific contributions but also that clinicians had the opportunity to spend an hour during an interview, describing in detail actions they took to protect patients from harm," said study lead author Alissa Russ-Jara, Ph.D., of Purdue University College of Pharmacy and U.S. Department of Veterans Affairs and a Regenstrief Institute affiliated scientist.
"By the end of the interview, many clinicians expressed surprise at how much nuance went into their own decision. Their decisions often occur so rapidly yet involve so much expertise. Ours was the first study to really unpack that for their decisions around drug-drug interactions."
"We expect our findings can improve the design and usability of drug-drug interaction alerts for clinicians and so they can more effectively aid patient safety. Our study focused on clinical decision-making, regardless of whether the clinician was warned by an alert or not, so our findings have implications for clinicians, informatics leaders, and patients, and for any EHR system."
Recommendations for alert design include:
- Provide information on the expected range of timing of potential drug-drug interaction effects (days, weeks, months or years)
- Provide a means for clinicians to review multiple electronic drug-drug interaction reference sources directly from the alert, side-by-side
- leverage data analytics to populate drug-drug interaction alerts with "smart" displays of alternative drugs, that align with three criteria used by clinicians.
- provide recommendations(s) on the alert along with associated patient characteristics (for example, "monitor, if patient indicates willingness and capability of measuring blood pressure daily")
"Cognitive Task Analysis of Clinicians' Drug-Drug Interaction Management during Patient Care and Implications for Alert Design" is published in BMJ Open.
More information: Alissa L Russ-Jara et al, Cognitive task analysis of clinicians' drug–drug interaction management during patient care and implications for alert design, BMJ Open (2023). DOI: 10.1136/bmjopen-2023-075512