This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Demystifying a key receptor in substance use and neuropsychiatric disorders
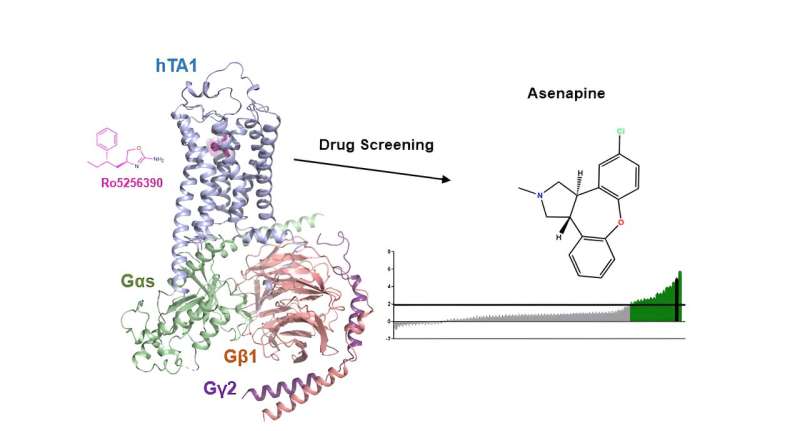
Researchers at the Icahn School of Medicine at Mount Sinai have uncovered insights into the potential mechanism of action of the antipsychotic medication asenapine, a possible therapeutic target for substance use and neuropsychiatric disorders. This discovery may pave the way for the development of improved medications targeting the same pathway.
Their findings, published in Nature Communications, show that a brain protein known as the TAAR1 receptor, a drug target known to regulate dopamine signaling in key reward pathways in the brain, differs significantly in humans compared to the preclinical rodent models on which drugs are typically tested.
The study suggests considering species-specific differences in drug-receptor interactions and further investigation into ways asenapine affects the body as steps toward potential therapeutic improvements.
"In investigating the functional and structural properties of TAAR1, our study aimed to shed more light on its mechanisms and pharmacology," says study first author Gregory Zilberg, a Ph.D. candidate at Icahn Mount Sinai. "Our findings may guide the development of novel TAAR1 drugs and prompt more exploration of medications similar to asenapine."
Using advanced techniques to investigate TAAR1's structure and function, the researchers identified three important elements. First, there are differences between rodent and human TAAR1 that likely affect how preclinical model studies can be translated to humans. Second, TAAR1 is much more closely related to serotonin and dopamine receptors than previously assumed. This suggests that several serotonin-targeting medications might have unknown therapeutic efficacy or side effects that are, in fact, due to their actions at TAAR1.
Finally, the investigators highlight that the clinically used antipsychotic asenapine unexpectedly shows strong activation of TAAR1, suggesting, in fact, that this serotonin- and dopamine-targeting antipsychotic could derive some of its therapeutic effects from TAAR1 activation.
If proven in further studies, this could open up new possibilities for its potential in other TAAR1-related therapeutic applications, such as its use in substance use disorders, as well as the development of new asenapine-based drugs.
The researchers noted the absence of information about differences in how TAAR1 works in rodents and humans and emphasized that some of these differences could account for why preclinical data on TAAR1 has not yet been successfully translated into effective therapies in humans. Next, the researchers plan to study where TAAR1 is located within cells and what its precise role is in influencing serotonin and dopamine signaling.
"This study provides a significant leap in understanding TAAR1, offering potential avenues for drug development and encouraging further research into its therapeutic applications," says senior author Daniel Wacker, Ph.D., Assistant Professor of Pharmacological Sciences, and Neuroscience, at Icahn Mount Sinai.
"As our work advances, we anticipate it may play a crucial role in shaping the development of new drugs targeting TAAR1 and offering valuable insights into how drugs similar to asenapine might work."
More information: Gregory Zilberg et al, Molecular basis of human trace amine-associated receptor 1 activation, Nature Communications (2024). DOI: 10.1038/s41467-023-44601-4