This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop framework to identify molecules responsible for chemotherapy resistance
Researchers at Karolinska Institutet and SciLifeLab demonstrate in a new study published in iScience how they can identify substances that can deactivate an enzyme responsible for chemotherapy resistance in cancer cells. These results could help improve chemotherapy effectiveness for cancer patients.
Chemotherapy is an important way in which we treat patients with cancer. But it is not always successful, as cancer cells can become resistant to these therapies. In an earlier study, the researchers identified an enzyme in cancer cells responsible for this therapy resistance, as this enzyme—called SAMHD1—could chemically inactivate the chemotherapy drugs.
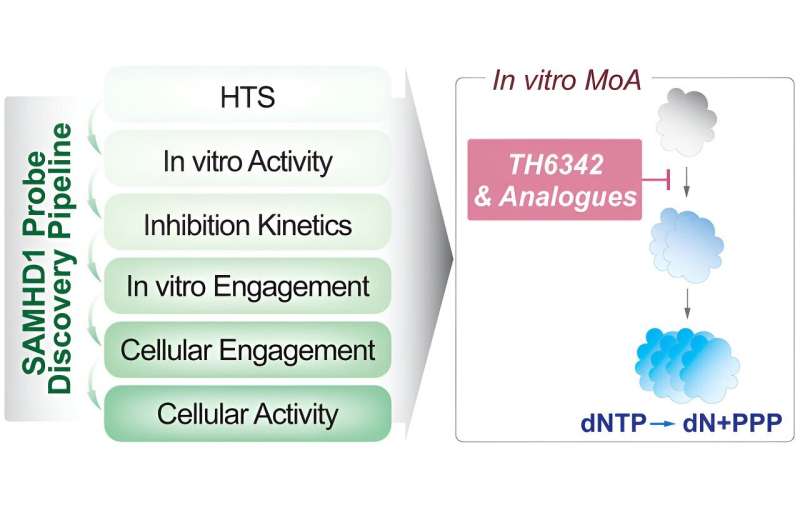
"This means that if we could develop a drug to switch off SAMHD1 in cancer cells we could potentially improve the effectiveness of chemotherapy treatments," says Assistant Professor Sean Rudd at the Department of Oncology-Pathology. "However, current efforts to do this are limited by a lack of available technologies to find and characterize such molecules. In the present study, we solve this problem, developing an experimental framework to find and characterize molecules able to directly switch off SAMHD1."
A thorough experimental framework
While we have a good understanding of the importance of the SAMHD1 enzyme in chemotherapy resistance, our current understanding of how to switch off this enzyme with small molecules is lacking. Having these molecules is an important step in translating important discoveries into the clinic.
Now our study provides a thorough experimental framework to the research community to find and characterize SAMHD1-inactivating small molecules. This is a critical step toward developing selective drugs capable of controlling SAMHD1 activity, which could be used, for instance, to enhance the effectiveness of chemotherapy treatments.
The study was co-led by Si Min Zhang and Sean Rudd, at the Department of Oncology-Pathology and SciLifeLab, and embraced a multidisciplinary approach encompassing biochemical and biophysical experiments on isolated protein together with experiments with cultured cancer cells.
The study contains a chemical screen against SAMHD1, an experiment in which more than 17,000 different small molecules are tested for their ability to inactivate this enzyme, which was done in collaboration with Chemical Biology Consortium Sweden at SciLifeLab. Collaboration with researchers, in particular medicinal chemists, in the group of Thomas Helleday (KI/SciLifeLab) was also an important component of the study.
Now an experimental framework is established to identify small molecules capable of inactivating SAMHD1 and understand the mechanism behind this process. The next step involves applying this knowledge practically. At present the researchers have several substances that they know inactivate SAMHD1. The researchers want to understand how these substances achieve this effect and utilize this knowledge for further improvement.
More information: Si Min Zhang et al, Identification and evaluation of small-molecule inhibitors against the dNTPase SAMHD1 via a comprehensive screening funnel, iScience (2024). www.cell.com/iscience/fulltext … 2589-0042(24)00128-7