This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Activity of pazopanib in EWSR1-NFATC2 translocation-associated bone sarcoma
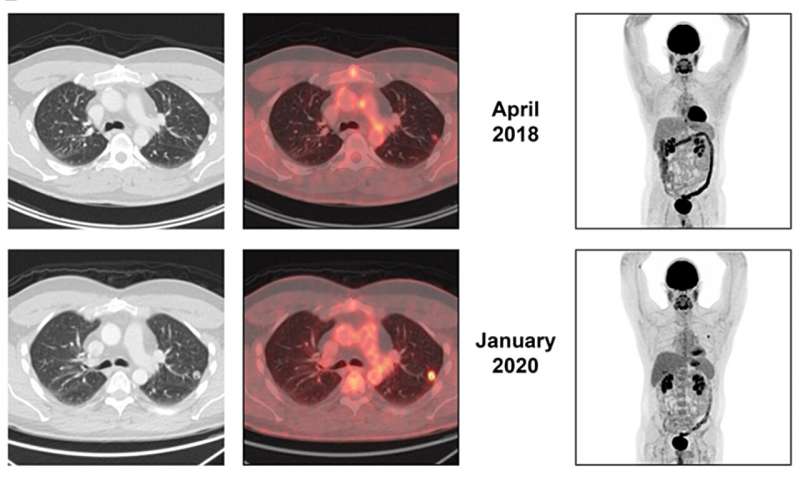
A new case report was published in Oncoscience on September 20, 2023, titled, "Activity of pazopanib in EWSR1-NFATC2 translocation-associated bone sarcoma."
In this case report researchers discuss the case of a patient with a EWSR1-NFATC2 fusion positive bone sarcoma who had exceptional tumor control through using pazopanib and surgery for an overall duration exceeding five years. The report also reviews the literature on EWSR1-NFATC2 translocation-associated sarcomas and use of pazopanib in bone sarcomas.
The researchers include Mohamed A. Gouda, Maria A. Zarzour, Ara A. Vaporciyan, Kalevi Kairemo, Hubert H. Chuang, and Vivek Subbiah from The University of Texas MD Anderson Cancer Center and Sarah Cannon Research Institute
Pazopanib, a multi-kinase VEGF inhibitor, is currently FDA approved for advanced renal cell carcinoma and advanced soft tissue sarcoma; but limited evidence exists on its efficacy in bone sarcomas.
"In brief, this case, in accordance with previously reported evidence, provides proof of activity of pazopanib in EWSR1-NFATC2 positive sarcoma. The report shows that pazopanib when administered in an adjuvant capacity demonstrated its effectiveness in preventing or delaying the progression of additional metastasis. Nevertheless, due to the adjuvant nature of the treatment, it remains uncertain whether this approach would have resulted in tumor shrinkage. Further preclinical studies and clinical studies using pazopanib in EWSR1-NFATC2 sarcomas are warranted," the researchers conclude.
More information: Mohamed A. Gouda et al, Activity of pazopanib in EWSR1-NFATC2 translocation-associated bone sarcoma, Oncoscience (2023). DOI: 10.18632/oncoscience.587