This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Delayed treatment effect predicting (DTEP) model found to enhance precision in immuno-oncology trial designs
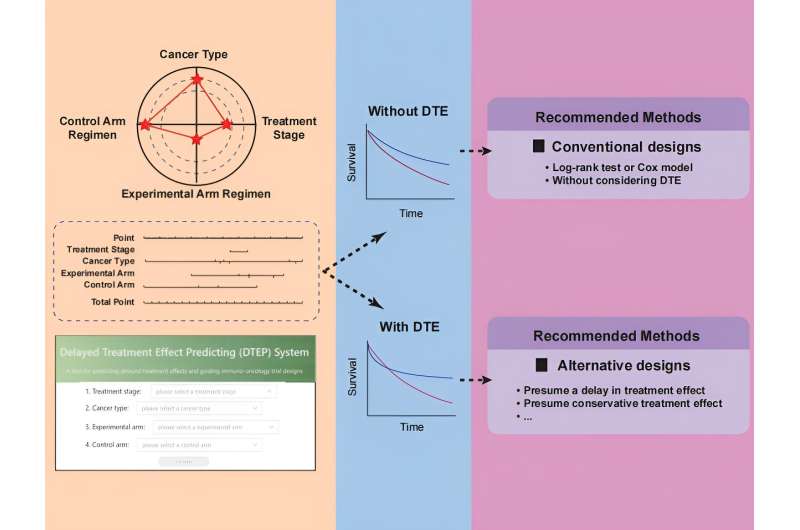
Over the last decade, immunotherapies, particularly immune checkpoint inhibitors (ICIs), have emerged as promising treatments for various cancer types. However, a notable challenge in immuno-oncology trials is the frequent occurrence of delayed treatment effects (DTE), where the therapeutic benefits of ICIs may take months to manifest.
Conventional trial designs often overlook the potential presence of DTE, leading to an underestimation of the required sample size and a loss of statistical power. Conversely, when there is no apparent delay in treatment effects, alternative trial designs addressing DTE may result in an overestimation of sample size and unnecessary trial prolongation.
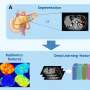
To address this critical challenge, a team led by Rui-Hua Xu, Ping-Yan Chen, Zi-Xian Wang, and colleagues developed the DTEP model. The model was trained and validated using data from 147 published randomized immuno-oncology trials. With this model, one can predict the DTE status using baseline characteristics, including cancer type, treatment line, and experimental and control arm regimens.
The DTEP model demonstrated high accuracy in predicting the DTE status, achieving an area under the operating characteristic curve (AUC) of 0.79 (95% CI, 0.71–0.88) in the training set and 0.78 (95% CI, 0.66–0.90) in the test set. Notably, the model successfully predicted the DTE status in two randomized trials (ESCORT-1st and JUPITER-02) among the test sets conducted by the research team.
To showcase the potential benefits of the DTEP model, the team simulated the re-conduction of the JUPITER-02 trial, where the DTE is recognized. The team used two alternative design approaches guided by the DTEP model and found that both designs improved statistical power and shortened trial durations compared to the conventional design.
In conclusion, the DTEP model presents a valuable tool for enhancing the precision and effectiveness of immuno-oncology trial designs. By predicting the presence of DTE, the model allows researchers to tailor trial designs, ultimately accelerating the development and approval of effective immunotherapies.
The research is published in the journal Medicine Plus.
More information: Zheng-Yu Qian et al, Delayed treatment effect predicting (DTEP) model for guiding immuno-oncology trial designs, Medicine Plus (2024). DOI: 10.1016/j.medp.2024.100006