This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Durvalumab and Tremelimumab before surgery in patients with HR+/HER2-negative breast cancer
A new research paper was published in Oncotarget entitled, "Durvalumab and tremelimumab before surgery in patients with hormone receptor positive, HER2-negative stage II–III breast cancer."
In this new study, researchers Haven R. Garber, Sreyashi Basu, Sonali Jindal, Zhong He, Khoi Chu, Akshara Singareeka Raghavendra, Clinton Yam, Lumarie Santiago, Beatriz E. Adrada, Padmanee Sharma, Elizabeth A. Mittendorf, and Jennifer K. Litton from the University of Texas MD Anderson Cancer Center, Brigham and Women's Hospital, Dana-Farber Brigham Cancer Center, and Harvard Medical School conducted a clinical trial to assess the feasibility of enrolling patients with Stage II or III hormone receptor-positive (HR+)/HER2-negative breast cancer to pre-operative dual PD-L1/CTLA-4 checkpoint inhibition administered prior to neoadjuvant chemotherapy (NACT).
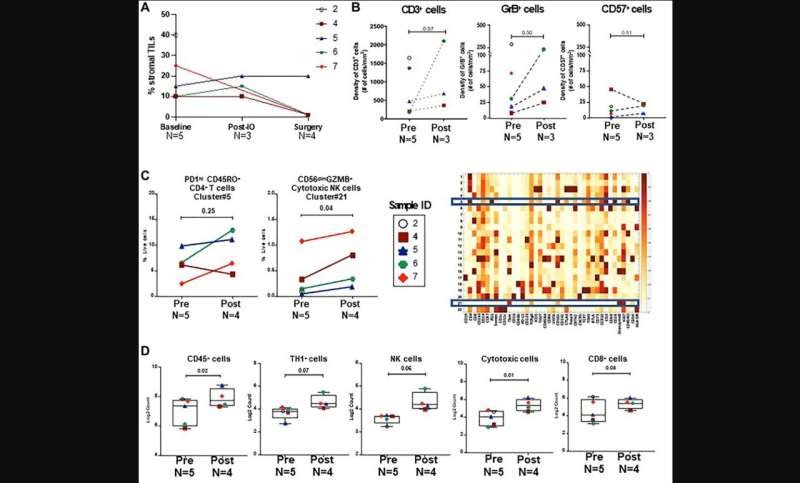
"This feasibility study was conducted to begin testing the hypothesis that dual checkpoint blockade would increase TIL and enhance the response to subsequent NACT in patients with stage II or III HR+/HER2-negative breast cancer."
Eight eligible patients were treated with upfront durvalumab and tremelimumab for two cycles. Patients then received NACT prior to breast surgery. Seven patients had baseline and interval breast ultrasounds after combination immunotherapy, and the responses were mixed: 3/7 patients experienced a ≥30% decrease in tumor volume, 3/7 a ≥30% increase, and 1 patient had stable disease. At the time of breast surgery, 1/8 patients had a pathologic complete response (pCR).
The trial was stopped early after 3 of 8 patients experienced immunotherapy-related toxicity or suspected disease progression that prompted discontinuation or a delay in the administration of NACT. Two patients experienced grade 3 immune-related adverse events (1 with colitis, 1 with endocrinopathy). Analysis of the tumor microenvironment after combination immunotherapy did not show a significant change in immune cell subsets from baseline.
"There was a limited benefit for dual checkpoint blockade administered prior to NACT in our study of 8 patients with HR+/HER2-negative breast cancer."
More information: Haven R. Garber et al, Durvalumab and tremelimumab before surgery in patients with hormone receptor positive, HER2-negative stage II-III breast cancer, Oncotarget (2024). DOI: 10.18632/oncotarget.28567

















