This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Using sacituzumab govitecan plus platinum-based chemotherapy in breast, bladder, and lung carcinomas
A new research paper titled "Sacituzumab govitecan plus platinum-based chemotherapy mediates significant antitumor effects in triple-negative breast, urinary bladder, and small-cell lung carcinomas" has been published in Oncotarget.
Sacituzumab govitecan (SG) is an antibody-drug conjugate composed of an anti-Trop-2-directed antibody conjugated with the topoisomerase I inhibitory drug, SN-38, via a proprietary hydrolysable linker. SG has received United States Food and Drug Administration (FDA) approval to treat metastatic triple-negative breast cancer (TNBC), unresectable locally advanced or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer, and accelerated approval for metastatic urothelial cancer (mUC).
In this new study, researchers Thomas M. Cardillo, Maria B. Zalath, Roberto Arrojo, Robert M. Sharkey, Serengulam V. Govindan, Chien-Hsing Chang, and David M. Goldenberg from Gilead Sciences and the Center for Molecular Medicine and Immunology investigated the utility of combining SG with platinum-based chemotherapeutics in TNBC, urinary bladder carcinoma (UBC), and small-cell lung carcinoma (SCLC).
"Given recent FDA approval of SG in mTNBC and accelerated approval in mUC [metastatic urothelial cancer], as well as its demonstrated clinical activity in SCLC, we investigated the possibility of expanding use of SG through combinations with currently utilized chemotherapeutics for these disease indications," the researchers explain.
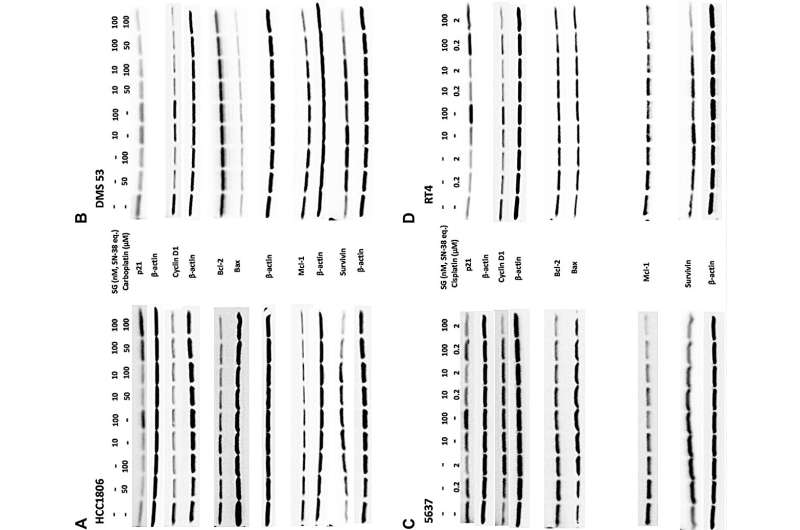
SG plus carboplatin or cisplatin produced additive growth-inhibitory effects in vitro that trended towards synergy. Immunoblot analysis of cell lysates suggests perturbation of the cell-cycle and a shift towards pro-apoptotic signaling evidenced by an increased Bax to Bcl-2 ratio and down-regulation of two anti-apoptotic proteins, Mcl-1 and survivin. Significant antitumor effects were observed with SG plus carboplatin in mice bearing TNBC or SCLC tumors compared to all controls (P < 0.0062 and P < 0.0017, respectively) and with SG plus cisplatin in UBC and SCLC tumor-bearing animals (P < 0.0362 and P < 0.0001, respectively). These combinations were well tolerated by the animals.
"Combining SG with platinum-based chemotherapeutics demonstrates the benefit in these indications and warrants further clinical investigation," the researchers conclude.
More information: Thomas M. Cardillo et al, Sacituzumab govitecan plus platinum-based chemotherapy mediates significant antitumor effects in triple-negative breast, urinary bladder, and small-cell lung carcinomas, Oncotarget (2024). DOI: 10.18632/oncotarget.28559