This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cockayne syndrome: New insights into cellular DNA repair mechanism
Cockayne syndrome is a severe autosomal recessive disorder caused by defective DNA repair mechanisms. People with the disease have much reduced life expectancy and suffer from facial deformities; growth failure; neurological, cognitive, and sensory impairments; bone, joint, and muscle deformities; kidney problems; and premature aging.
Like xeroderma pigmentosum (XP), Cockayne syndrome (CS) is a disease where elements of nucleotide excision repair (NER) do not work properly. The purpose of this repair mechanism is to remove DNA damage caused by ultraviolet (UV) light, chemicals, and various other environmental factors.
Researchers from the group of biochemist Professor Julian Stingele from LMU's Gene Center Munich have now uncovered important details about the role of the CSB/ERCC6 and CSA/ERCC8 genes involved in Cockayne syndrome. These genes encode two enzymes associated with DNA repair.
The results of their work have been published in the journal Nature Cell Biology.
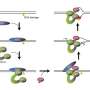
"Our data point to a new, previously unknown function of these two genes and their gene products in the repair of covalent DNA-protein interactions in the course of transcription," reports Stingele, referring to the cytotoxic, biologically undesirable crosslinking of proteins to DNA.
An obstacle for transcription
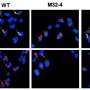
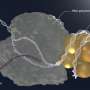
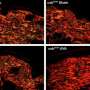
In collaboration with researchers from the University of Cambridge, the scientists demonstrated that DNA-protein crosslinks present a physical obstacle to further transcription. Arresting transcription brings CS proteins to the blockade sites.
"Our results indicate that CSB and CSA then initiate the transcription-coupled repair of the toxic DNA-protein crosslinks," says Stingele. "This previously unrecognized cellular function of CS proteins leads to the marking of the DNA damage—and thence to its enzymatic breakdown."
The study also revealed that this newly discovered function of CS proteins works independently of classic TC-NER (transcription-coupled nucleotide excision repair) enzymes, which are deployed, among other things, for repairing DNA damage caused by UV light—and the absence of which leads to xeroderma pigmentosum.
"The fact that CS proteins have additional functions is noteworthy. This discovery could help to explain the pathological differences between xeroderma pigmentosum and Cockayne syndrome," says Stingele. CS is a more severe and more multifaceted disorder than XP, with complex and incompletely understood causes.
As their next step, Stingele's research group plans to decode the exact process of CS-protein-mediated repair.
More information: Christopher J. Carnie et al, Transcription-coupled repair of DNA–protein cross-links depends on CSA and CSB, Nature Cell Biology (2024). DOI: 10.1038/s41556-024-01391-1