This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Novel pre-clinical models help advance therapeutic development for antibiotic-resistant bacterial infections
The A*STAR Infectious Diseases Labs (A*STAR ID Labs) and the Hackensack Meridian Health Center for Discovery and Innovation have teamed up to shed light on a concerning health issue—infections caused by a type of bacteria known as Klebsiella pneumoniae (Kp).
Utilizing innovative pre-clinical models that mimic disease progression in the human body, the research team revealed key distinctions in the behavior of various Kp strains, enabling the development of targeted treatment strategies or alternative intervention approaches for Kp-related diseases in Asia.
Kp is responsible for the highest proportion of antibiotics resistant infections in the world, accounting for about 80 percent in the U.S. and about 40 percent in Asia, and can cause infections in the lungs (pneumonia), urinary tract (UTI) and other organs. It is a critical priority bacterium in urgent need of new treatments, as classified by the World Health Organization (WHO).
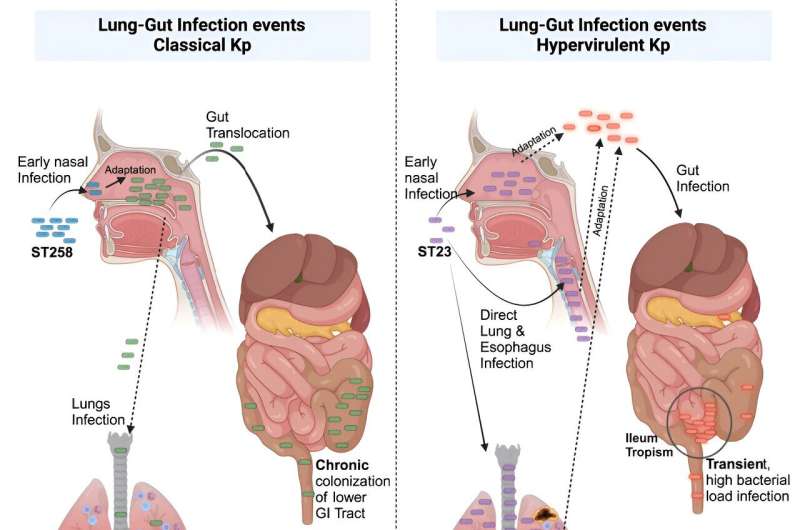
By creating infection models that closely resemble the real-life disease progression in humans, the research team uncovered crucial differences in the behavior of the type of Kp responsible for hospital-acquired infections (dominant in the U.S.), in contrast to its counterparts responsible for community-acquired infections (dominant in Asia). This has provided insights to understand better the differences in the clinical outcomes of the two dominant types of Kp, offering hope for improved strategies to combat Kp-related diseases.
The paper "Differential mucosal tropism and dissemination of classical and hypervirulent Klebsiella pneumoniae infection" was published in iScience.
Antibiotic-resistant bacteria have always been a major health care issue globally. In 2019, a study conducted estimated that the actual mortality burden of drug-resistant bacterial infection accounted for 4.95 million deaths. Research has shown that Kp was one of the top three causes of these deaths, accounting for the highest proportion of antibiotic-resistant infections.
In Singapore, infections resistant to antibiotics were the fourth leading cause of death from diseases, with Kp responsible for about 43 percent of infections that could not be treated with strong antibiotics.
While Kp has been a significant threat to health care, it continues to fly under the radar as the research community struggles to understand how it causes diseases. Additionally, there has also been a lack of comparative studies on the differences between Kp observed in the West and Asia, where Kp management strategies adopted may differ.
To address these research gaps, the research team developed novel and comparable infection models for both dominant types of Kp that closely mimic the actual onset of infection and how the bacteria spreads in the body. These pre-clinical models are critical for examining how each Kp type behaves in the nose, lungs, and digestive system.
The team found notable differences in aggressiveness between the hospital-acquired strains and the community-acquired strains. The Kp strains commonly found in the communities (outside hospitals) reported in Asia are more aggressive, multiplying directly in the lungs to dangerous levels and starting infection in the throat, which then moves to parts of the digestive system temporarily, causing a more severe infection.
Currently, the treatments available to fight Kp infections are limited, raising an urgent need to consider alternative interventions such as vaccinations and phage therapy such as using viruses that target bacteria and host-directed therapy.
The novel pre-clinical testing models developed in this study also serve as a platform for clinicians and the scientific community to easily assess the effectiveness of novel drugs and interventions and to develop targeted treatment strategies for the different types of Kp.
Dr. Teck Hui Teo, Investigator at A*STAR ID Labs and co-lead of this study, said, "Our bodies' mucosal tissues are home to the most microorganisms, yet we're just beginning to understand how these microbes interact with these tissues."
"This study reveals how the bacterium Kp can take over the tissue in the nasal cavity to colonize the gut later, turning it into a hub for further spread. Unique to the Asian isolates, we saw aggressive infection outcomes in the lung and gut that required our attention. We aim to use this knowledge to target such adaptation events and develop targeted treatments in the future."
Prof Pablo Bifani, Senior Principal Investigator at A*STAR ID Labs, senior author, and co-lead of this study, said, "Creating novel pre-clinical models has helped us understand how the different types of drug-resistant Kp behave differently. As the hospital-acquired Kp strains and the community-acquired Kp strains are dominant in different parts of the world, it highlights the importance of studying these bacteria more closely, taking into account the differences found in various regions."
More information: Teck-Hui Teo et al, Differential mucosal tropism and dissemination of classical and hypervirulent Klebsiella pneumoniae infection, iScience (2024). DOI: 10.1016/j.isci.2024.108875