This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
A boost for HIV vaccine research: Studies present comprehensive platform for validating next steps
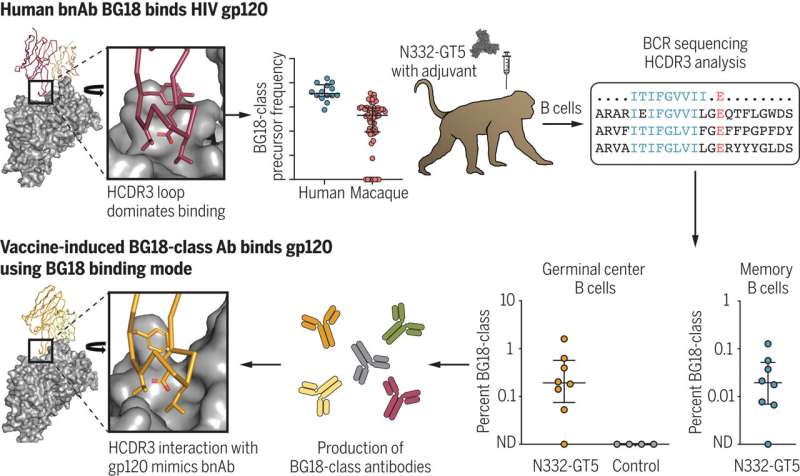
HIV has proven a hard target for vaccine design. The most promising approach, germline-targeting (GT), proposes a series of immunizations: a first shot to activate inexperienced B cells—antibody-producing white blood cells—followed by a sequence of immunogens that are more and more like the HIV Envelope (Env) protein.
The ultimate goal of GT immunization is to coach B cells into producing broadly neutralizing antibodies (bnAbs) capable of binding to conserved sites on HIV Env, which seldom change despite HIV's penchant for rapid diversification.
A substantial amount of research has demonstrated the feasibility of the initial activation step; now, in two papers simultaneously out in Science and Science Immunology, researchers at the Ragon Institute of Mass General, MIT, and Harvard have developed a comprehensive platform for HIV vaccine research capable of both preclinically validating next-step boost immunogens and providing new insights into the basic biology of the antibody response.
These articles have been published in tandem with manuscripts from the Scripps Research Institute group in Science and Science Translational Medicine. This suite of four papers is the outcome of years of intense collaboration between the institutes and represents a major step towards an HIV vaccine.
"The key tool for our HIV program is the humanized mouse model," explains Professor Facundo D. Batista, Ph.D., Associate and Scientific Director of the Ragon Institute and PI of the lab in which the mouse studies were conducted.
"With knock-in human B cell receptors identified as potential bnAb precursors, we can then observe how they respond to immunogens as part of a complete mammalian immune system. We've used a CRISPR-based approach to develop mouse lines to interrogate several known conserved sites on the HIV Envelope for which our collaborators at Scripps have developed immunogens."
While first-author research scientists Zhenfei Xie, Ph.D., and Xuesong Wang, Ph.D., both carried out the work underpinning their respective Science and Science Immunology articles in the Batista lab at the Ragon Institute, each focused on a different conserved site on the HIV Env and a different aspect of the fundamental biology of immunization. Both scientists, however, came to a similar conclusion about delivery format: the mRNA-LNP system made famous by the Pfizer and Moderna COVID-19 vaccines was highly effective for HIV boost immunogens.
For Dr. Xie, the consilience between the work performed by the immunogen design team at Scripps, led by Prof. William Schief, and the immunobiological work in the Batista lab was the key factor.
"bnAbs against HIV-1 are the uncommon outcome of a long journey in HIV-infected patients. Jon Steichen worked backwards from the co-evolutionary trajectory of the bnAb and the virus, understanding how their structures interact, and then we moved everything back into an in vivo model where we could start observing messier phenomena like antibody competition. That collaboration was central to demonstrating that these types of very site-specific boosts can work."
The preclinical platform can address more fundamental biological problems. B cell lines that might develop bnAbs are often rare in humans, and one way the Scripps team has sought to overcome that difficulty is by designing immunogens capable of engaging a wide array of B cell lineages with that potential—but would those B cells compete, limiting immunogen effectiveness?
Dr. Wang produced a model with multiple potential precursors to CD4 binding site (CD4bs) bnAbs.
"The mRNA-LNP prime-boosts Christ Cottrell developed not only induced multi-lineage precursor B cell responses, but also triggered bnAb-like affinity maturation," she says. "Multiple lines could be matured in the same mouse at the same time."
Now, the challenge will be found in combining their work. "We're at the stage where there are an increasing number of immunogens in the pipeline targeting different Env sites," says Prof. Batista.
"Ultimately, our hope is to bring these projects together and start to understand the best way to generate a response to multiple sites: that's when you start really clamping down on the potential for viral escape. We already have this work underway."
More information: Zhenfei Xie et al, mRNA-LNP HIV-1 trimer boosters elicit precursors to broad neutralizing antibodies, Science (2024). DOI: 10.1126/science.adk0582
Xuesong Wang et al, mRNA-LNP prime boost evolves precursors toward VRC01-like broadly neutralizing antibodies in preclinical humanized mouse models, Science Immunology (2024). DOI: 10.1126/sciimmunol.adn0622
Jon M. Steichen et al, Vaccine priming of rare HIV broadly neutralizing antibody precursors in nonhuman primates, Science (2024). DOI: 10.1126/science.adj8321
Christopher A. Cottrell et al, Heterologous prime-boost vaccination drives early maturation of HIV broadly neutralizing antibody precursors in humanized mice, Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.adn0223
Rogier W. Sanders et al, Progress on priming HIV-1 immunity, Science (2024). DOI: 10.1126/science.adp3459