This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Early antibiotic use linked to 'microbial scar' in preterm infants
According to a new publication in Nature Communications, early antibiotics and prolonged hospitalization leave a "nasopharyngeal microbial scar" in preterm infants. Results underscore the critical role of antibiotic stewardship and infection control within neonatal intensive care units, highlighting the need to balance life-saving antibiotic use with the potential impact on the developing nasopharyngeal microbiome.
Preterm infants are especially vulnerable to infections, often necessitating early antibiotic interventions to ward off infections. However, a new groundbreaking study titled "Prolonged hospitalization signature and early antibiotic effects on the nasopharyngeal resistome in preterm infants" reveals that these life-saving measures come with significant effects on the infants' nasopharyngeal microbiomes ("microbial scar").
Researchers from the University of Oslo and Oslo University Hospital, together with collaborators at the Norwegian Institute of Public Health and Rigshospitalet in Denmark, conducted a prospective cohort study to investigate how early antibiotic exposure impacts the development of antibiotic resistance in the nasopharynx of preterm infants. The study also investigates how these changes correlate with various clinical factors, filling a critical gap in our understanding of the nasopharyngeal microbial communities in this vulnerable population.
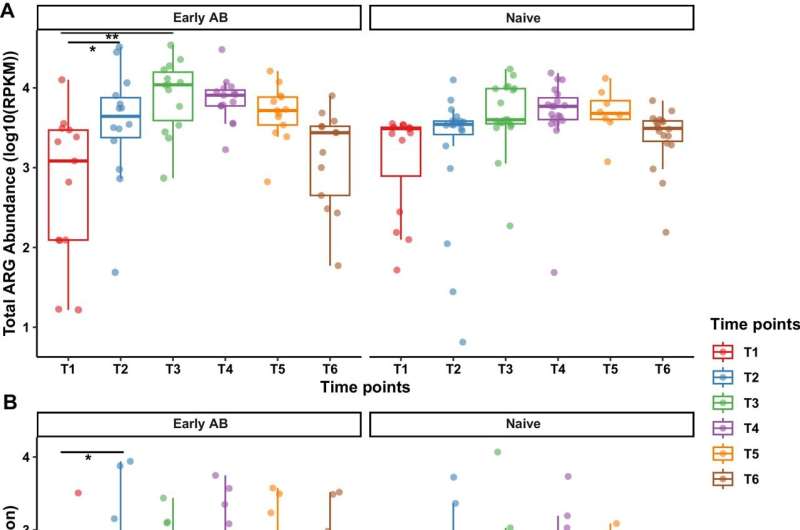
The study involved 198 nasopharyngeal aspirate samples from 36 preterm infants, born between 28 and 32 weeks of gestational age, at the Ullevål Neonatal Intensive Care Unit at Oslo University Hospital. These infants were divided into two groups: those who received antibiotics within the first 24 hours of life due to an increased risk for early-onset neonatal sepsis (EONS), and those who did not receive antibiotics.
The samples were collected from birth up to 8–10 months of age, and shotgun metagenomics was employed to characterize and analyze the landscape of antibiotic resistance genes (ARGs) i.e., resistome, present in these microbial communities. This comprehensive methodological approach had been explored in a previous study with a smaller sample size, but it was uncertain whether it would be effective with the larger sample size used in this study. However, the pioneering methodology proved robust, allowing in-depth sequencing and analysis.
While early antibiotic treatment did cause transient disruptions in the nasopharyngeal resistome, the overall diversity and abundance of ARGs did not significantly differ between treated infants and the control group by 8-10 months of life. However, a critical observation was the persistence of ARGs associated with the nosocomial pathogen Serratia marcescens, which remained in the nasopharynx of 92% of affected infants long after hospital discharge.
Dr. Dhariwal, who conducted the bioinformatic analysis, stated, "Our findings underscore the importance of hospital environments in shaping the preterm infants' resistome."
Contrary to earlier studies on gut resistomes, which showed long-term colonization by antibiotic-resistant bacteria post-treatment, this study found no lasting alterations in the nasopharyngeal resistome beyond the early months of life. Nonetheless, the consistent presence of resistance genes associated with Serratia marcescens highlights the lingering impact of hospitalization.
"We did not expect such a pronounced signature from a single organism," noted Dr. Petersen, senior author and Professor at the University of Oslo.
This study is among the first to comprehensively detail the nasopharyngeal resistome and microbiome of preterm infants. While the same research group had previously tested their methodologies on fewer samples, this study confirms their efficacy on a larger scale, setting a new benchmark for future research in this field.
Investigating the nasopharyngeal microbiome is especially important because it serves as a key pathway for infections and a reservoir for drug-resistant pathogens. Additionally, the respiratory tract microbiome functions as a gatekeeper to health by inhibiting pathogen growth and communicating with the gut microbiome to regulate immunity.
Although highly significant, this field has received less attention, partly due to technical hurdles that emerging metagenomic techniques, as demonstrated by this group's optimized methodologies, are now overcoming.
The group's findings emphasize the importance of antibiotic stewardship and robust infection control measures in NICUs. While Norway boasts stringent controls and low levels of antimicrobial resistance (AMR), the risks highlighted by this study underscore the need for vigilance in all health care settings.
"Antibiotics are invaluable, but their use must be judicious," said Dr. Petersen. "In countries with high levels of antimicrobial resistance, the implications for preterm infants could be more pronounced, potentially affecting their health outcomes."
Additionally, the study's methodological advancements pave the way for more extensive research into nasopharyngeal or other respiratory niches, providing a new protocol for future studies on the immediate and long-term effects of antibiotic exposure.
Dr. Petersen concluded, "By establishing these methods, we hope to inspire further investigations into the carriage of antibiotic resistance by microbes in the respiratory tract, ultimately leading to improved outcomes for the most vulnerable patients."
More information: Achal Dhariwal et al, Prolonged hospitalization signature and early antibiotic effects on the nasopharyngeal resistome in preterm infants, Nature Communications (2024). DOI: 10.1038/s41467-024-50433-7