This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Team reveals why new tuberculosis vaccine induces a stronger, longer response than the conventional vaccine

Researchers from the Butantan Institute and collaborators are developing a more potent version of the BCG vaccine that protects against tuberculosis. While the conventional immunizer reduced infection by 90% in experiments with mice, the so-called recombinant BCG increased the protection rate to 99%. In addition, the new formulation protected the animals for a significantly longer period of time.
"BCG is the first vaccine we receive at birth, and it's indeed effective in protecting children. But immunity against the disease tends to wane in adulthood, and as bacteria are becoming resistant to antibiotics, no one is safe. There's been a global effort to try to improve the prevention of pulmonary tuberculosis in adults. Today, there are about 10 million new cases and 1.5 million deaths per year in the world," said Agência FAPESP Luciana Cezar de Cerqueira Leite, a researcher at Butantan Institute's Vaccine Development Laboratory.
To understand why recombinant BCG leads to a stronger and longer-lasting immune response, the Butantan group and collaborators from several countries have adopted an approach known as systems biology—in short, observing in an animal model the behavior of thousands of genes in different tissues (mainly the lungs and lymph nodes) throughout the assembly of the immune response. This involves sequencing hundreds of samples, followed by intensive bioinformatics and data mining.
Cerqueira Leite addressed this topic on June 29 during a presentation at FAPESP Week China. The panel, dedicated to health and biomedicine issues, also included Zhang Zhiyong of Guangzhou Medical University, Pedro Moraes-Vieira of the State University of Campinas (UNICAMP), Xin Jin, chief research scientist at the Chinese company BGI, and Dan Zhang, co-founder of the company Hillgene BioPharma, also from China. The mediators were Xin Jin (BGI) and Simone Appenzeller, a professor at UNICAMP.
"We sequenced all the RNA that was expressed and present in samples taken at different times: before the animal was immunized, seven and 90 days after immunization—when the challenge is performed [the bacteria are inoculated into the rodents' noses]—and seven and 90 days after the challenge," explains Leite.
Samples from three groups of mice were compared: one was not immunized, another received the conventional BCG, and a third was vaccinated with recombinant BCG. At each moment of analysis, the genes with increased or decreased expression in the different groups were compared.
"As early as seven days after immunization, a very big change could be seen in the group that received the recombinant version: about 200 genes were already being activated, while almost nothing happened in the conventional BCG group. After 90 days, the response to the conventional BCG began to decline, while it remained the same in the recombinant group. But the big news is that the two vaccines work through very different metabolic pathways and we've been able to map them. The data will be published soon," reveals the researcher.
Turbocharged bacteria
The BCG vaccine, ubiquitous in Brazilian maternity wards, was developed over a hundred years ago by attenuating the bacterium that causes bovine tuberculosis (Mycobacterium bovis), which is very similar to that of human tuberculosis (M. tuberculosis).
Through genetic engineering, the recombinant version gained the ability to produce a fragment of a toxin originally secreted by Escherichia coli, a species commonly found in the human gut.
"Some E. coli bacteria produce heat-labile toxins, known by the acronym LT, which are highly immunogenic, i.e. they induce a strong response from the immune system. In the recombinant vaccine, we use a detoxified fragment of the LT toxin [called LTAK63]. It isn't capable of causing disease, but it maintains immunogenicity, so it acts as an adjuvant to the BCG vaccine," Leite explains.
The first studies using the recombinant BCG strategy were aimed at developing a neonatal pertussis vaccine, the researcher explained. "This is because BCG can be given at birth, while the currently available pertussis immunizer [the triple bacterial vaccine, or DTP] provides protection only from the age of six months. In this case, we use a fragment of the toxin produced by the pertussis bacterium [Bordetella pertussis]," she explains.
Given the promising results, the same platform has been used in the search for new and better vaccines against whooping cough, tuberculosis, pneumonia (caused by Streptococcus pneumoniae or pneumococcus) and schistosomiasis, as well as in the treatment of bladder cancer (in which case the vaccine is used to "wake up" the immune system and stimulate it to attack the tumor).
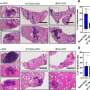
In experiments with an animal model of tuberculosis, recombinant BCG was found to reduce the amount of bacteria in the lungs a hundredfold and also to reduce inflammation in the organ.
"In trying to kill the bacteria, the defense cells end up attacking everything nearby and causing tissue damage. But the lungs of the animals immunized with recombinant BCG are white and less inflamed. By detailing the mechanism of action of the two vaccines, we now understand why this happens," says Leite.
All RNA sequencing was performed in Shenzhen, China, by the company BGI, which provides genomics services to laboratories all over the world. Researchers from the Shenzhen Institute of Advanced Technology, the University of São Paulo (USP), the Albert Einstein Israelite College of Health Sciences, Fiocruz Bahia, Novartis/GSK, the Institut Pasteur and the Federal University of Goiânia (UFG), among others, collaborated in the studies.