Two medications from one manufacturer recalled for a failure that can cause heart attacks
A total of 135 batches of potassium chloride capsules have been recalled because the extended release capsules might not release.
Jul 1, 2024
0
1
A total of 135 batches of potassium chloride capsules have been recalled because the extended release capsules might not release.
Jul 1, 2024
0
1

Potassium channels are openings that allow charged potassium atoms to cross the cell membrane. Voltage-gated potassium channels—which open only when a specific voltage is reached across the cell membrane—are essential ...
Jun 27, 2024
0
52

In Greek mythology, a water nymph's curse forces a man to stay awake or suffocate. For a rare segment of the population today, the curse is all too real. Now, a team led by University of Connecticut researchers describes ...
Jun 25, 2024
0
63

Researchers from Duke-NUS Medical School have found that a new class of light-sensitive proteins are capable of turning off brain cells with light, offering scientists an unprecedentedly effective tool to investigate brain ...
May 24, 2024
0
26

In a groundbreaking discovery, scientists at the Natural History Museum of Los Angeles County and pharmaceutical firm AstraZeneca have published the first-ever mineral-based treatment for a widespread disease.
Mar 27, 2024
0
126
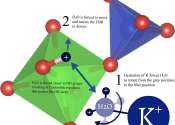
Researchers co-led by Nanyang Technological University, Singapore (NTU Singapore) and the University of Toulouse, France, have uncovered how bacteria and their toxins prompt the human immune response, leading to inflammation.
Mar 20, 2024
0
33

A five-day course of once-daily inorganic nitrate reduces the risk of a serious complication following a coronary angiogram, in which the dye used causes damage to the kidneys. The clinical trial, led by Queen Mary University ...
Mar 18, 2024
0
1
While reducing salt intake has been the focus of dietary advice to control high blood pressure (hypertension), a new study suggests that upping your potassium intake can be at least as important.
Feb 21, 2024
0
51

Brain function depends on the swift movement of electrical signals along axons, the long extensions of nerve cells that connect billions of brain cells. The nerve fibers are insulated by a fatty layer called myelin, which ...
Jan 31, 2024
0
55

A call to include recommendations on low-sodium potassium-enriched salt in hypertension treatment guidelines has been made by an international group of experts in the journal Hypertension.
Jan 25, 2024
0
11

Potassium (pronounced /pɵˈtæsiəm/) is the chemical element with the symbol K (Latin: kalium, from Arabic: القَلْيَه al-qalyah “plant ashes”, cf. Alkali from the same root), atomic number 19, and atomic mass 39.0983. Potassium was first isolated from potash. Elemental potassium is a soft silvery-white metallic alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the evolved hydrogen.
Potassium in nature occurs only as ionic salt. As such, it is found dissolved in seawater, and as part of many minerals. Potassium ion is necessary for the function of all living cells, and is thus present in all plant and animal tissues. It is found in especially high concentrations in plant cells, and in a mixed diet, it is most highly concentrated in fruits.
In many respects, potassium and sodium are chemically similar, although they have very different functions in organisms in general, and in animal cells in particular.
This text uses material from Wikipedia, licensed under CC BY-SA