Timothy syndrome mutations provide new insights into the structure of L-calcium channel
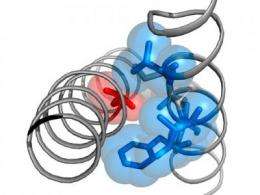
The human genome encodes 243 voltage-gated ion channels. Mutations in calcium channels can cause severe inherited diseases such as migraine, night blindness, autism spectrum disorders and Timothy syndrome, which leads to severe cardiovascular disorders. Katrin Depil and Anna Stary-Weinzinger together with colleagues from the Department of Pharmacology and Toxicology, University of Vienna analyzed changes in molecular organization of calcium channels caused by Timothy syndrome mutations. Recently, they published their current research results in the Journal of Biological Chemistry.
Ion channels are large membrane proteins that conduct potassium, sodium or calcium ions. They regulate electrical signals in the nervous system, control the release of neurotransmitters and are responsible for the regulation of the heart rhythm and muscle contractions. Voltage-gated calcium channels, like all other voltage-gated ion channels open and close in response to changes in membrane potential. The exact mechanisms underlying this gating process are still unexplored. It is however known that mutations can severely affect channel opening and closing, thereby disturbing the calcium homeostasis, which could lead to so called "ion channel diseases" or "channelopathies".
Life-threatening disease
Timothy syndrome, which was first described in the 90s, often leads to sudden cardiac death in early childhood. In 2004 it was discovered that mutations in calcium channel, which replace two amino acids in the ion channel protein sequence with other amino acids, cause the neurological disorders, autism, severe arrhythmias and webbing of fingers and toes that are associated with the Timothy syndrome. Prof. Hering, Head of the Department of Pharmacology and Toxicology of the University of Vienna, explains that the Timothy mutations result in enhanced calcium entry caused by defects in channel closure during an action potential. This in turn induces a calcium overflow causing arrhythmias and multiple disease patterns."
Destabilization of the closed pore
The current research focus of the two young scientists, Katrin Depil and Anna Stary-Weinzinger, are voltage gated calcium channels. In the recently published paper in Journal of Biological Chemistry the authors describe that the Timothy-mutation is part of a highly conserved structure motif, which consists of small amino acids – glycines (G) and alanines (A), which they named the "G/A/G/A"-motif. The strongest effect on channel opening occurs when residues from this motif are replaced with bigger hydrophobic amino acids. Anna Stary-Weinzinger: "We assume that the Timothy G406 and the whole G/A/G/A-motif are essential for sealing of the closed channel pore. Mutations to larger amino acids in this position prevent optimal channel closure. Our data suggest that these residues form an important part of the channel gate."
Guided by systematic mutation and correlation analyzes of specific pore segments in calcium channels, Katrin Depil already succeeded in identifying key amino acid side chain properties, that play a key role in the molecular mechanism of channel opening and closure. Katrin Depil: "By analyzing further interactions in different positions in the pore region we aim to refine our calcium channel homology models. We hope to contribute to a better understanding of Timothy disease and other channelopathies."
More information:
Depil K, Beyl S, Stary-Weinzinger A, Hohaus A, Timin E, Hering S. Timothy mutation disrupts link between activation and inactivation in CaV1.2. Journal of Biological Chemistry. Jun 17, 2011
DOI: 10.1074/jbc.M111.255273