Women exposed to diethylstilbestrol in the womb face increased cancer risk
A large study of the daughters of women who had been given DES, the first synthetic form of estrogen, during pregnancy has found that exposure to the drug while in the womb (in utero) is associated with many reproductive problems and an increased risk of certain cancers and pre-cancerous conditions. The results of this analysis, conducted by researchers at the National Cancer Institute (NCI), part of the National Institutes of Health, and collaborators across the country, were published Oct. 6, 2011, in the New England Journal of Medicine.
Beginning in 1940, diethylstilbestrol, known as DES, was used clinically to prevent certain complications of pregnancy. In the 1950s, clinical studies showed DES was ineffective for this purpose. In the late 1960s, an unusual occurrence of a rare cancer of the vagina among young women, called clear cell adenocarcinoma (CCA), was observed and subsequently linked to their exposure to DES while in the womb.
In 1971, the U.S. Food and Drug Administration notified physicians that DES should not be prescribed to pregnant women. However, between 5 million and 10 million pregnant women and babies had been exposed to the drug. It was manufactured under many different product names, and came in various forms, including pills, creams and vaginal suppositories.
"Our study carefully documents elevated risk for DES-exposed daughters for a host of medical problems -- many of them also quite common in the general population," said study author Robert N. Hoover, M.D., director of the Epidemiology and Biostatistics Program in NCI's Division of Cancer Epidemiology and Genetics. "Without the sentinel finding of a very rare cancer in young women, and without the sustained follow-up of those who were exposed, we would not know the full extent of harm caused by DES exposure in the womb."
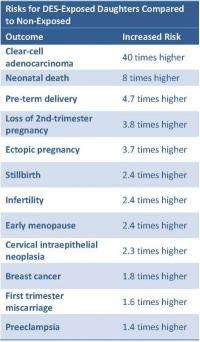
In this study, which included over 6,500 women (4,600 exposed and 1,900 unexposed), the researchers found that the daughters with exposure to DES while in the womb had an increased risk of 12 medical conditions, including a twofold higher risk of infertility and a fivefold increased risk of having a preterm delivery. (See table below for complete list of increased risks.)
This study is also the first to estimate the cumulative proportion of all DES-exposed women who developed these conditions because of their exposure. Of all DES-exposed women, 1 in 5 will experience some level of infertility because of their exposure. And of all those exposed women who are successful in having at least one birth, 1 in 3 will have a preterm delivery due to DES.
Although DES-exposed daughters have about 40 times the risk of developing CCA than unexposed women, CCA is still a rare disease, occurring in 1 in 1,000 DES-exposed daughters. While the first women diagnosed with this condition in the late 1960s were adolescents and young adults at the time of their diagnosis, the research now shows that the risk for DES-exposed daughters continues through at least age 40. In addition, these women are more than twice as likely to develop pre-cancerous cells in the cervix or vagina (called cervical intraepithelial neoplasia) and have an 80 percent higher chance of developing breast cancer after age 40. According to the results of this study, by age 55, 1 in 25 DES-exposed daughters will develop abnormal cellular changes in the cervix or vagina, and 1 in 50 will develop breast cancer due to their DES exposure.
This study was the first to assess risk based on the presence of vaginal epithelial changes as a biomarker of timing and dose of DES exposure. Exposed daughters with higher total dose of DES and younger age of the embryo at first exposure had evidence of these changes in the lining of the vagina. Women with these changes were at even greater risk for 9 of the 12 conditions compared to exposed women who did not have the biomarker.
This study did not evaluate sons with DES exposure in the womb, but previous reports have indicated an increased risk for certain testicular abnormalities, including undescended testicles or the development of cysts in the epididymis, tightly coiled tubes connected to the testicles. As DES-exposed sons grow older, more data will be available to assess their cancer risk. So far, research has shown no decreased fertility for these men, even with testicular abnormalities.
The women in this study were followed as part of the NCI's DES Follow-up Study, which began in 1992. NCI researchers will continue to study DES-exposed daughters as they move into menopausal years. The cancer risks for exposed daughters, as well as sons, are continually being studied to determine if they differ from an unexposed population. In addition, researchers are studying possible health effects on the grandchildren of mothers who took DES during pregnancy, because some of the genetic changes caused by DES exposure in the womb may be inherited.














