KS-herpesvirus induces reprogramming of lymphatic endothelial cells to invasive mesenchymal cells
Kaposi's sarcoma herpesvirus (KSHV) is an etiological agent for Kaposi's sarcoma and two other rare lymphoproliferative malignancies, and it is the most common cancer in HIV-infected untreated individuals. Researchers at the University of Helsinki, Finland, have discovered a novel viral oncogenesis mechanism in which KSHV oncogenes co-opt cellular signaling pathways and modify the cellular microenvironment more permissive for viral replication. The study will be published Dec. 15, 2011, in Cell Host & Microbe.
Human tumor viruses contribute to 15-20% of human cancers worldwide. Kaposi's sarcoma herpesvirus (KSHV) is an etiological agent for Kaposi's sarcoma (KS) and two other rare lymphoproliferative malignancies. KS is the most common cancer in HIV-infected untreated individuals and remains a primary cause of cancer deaths in many subequatorial African countries as a result of the AIDS pandemic. Researchers at the Institute of Biotechnology and Research Programs Unit (Genome-Scale Biology) at the University of Helsinki have discovered a novel viral oncogenesis mechanism in which KSHV oncogenes co-opt cellular signaling pathways and modify the cellular microenvironment more permissive for viral replication.
The findings by the group of Research Professor Päivi Ojala (Institute of Biotechnology, University of Helsinki) demonstrates the first lymphatic-specific endothelial-to-mesenchymal transition (EndMT) induced by a human tumor virus. The virus-induced EndMT can contribute to development of KS by giving rise to infected, invasive cells, and providing the virus a permissive cellular microenvironment for efficient spread of the virus.
"This information can be used for developing targeted therapies to prevent or at least slow down the progression of KS in immunosuppressed patients", Dr. Ojala says.
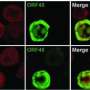
By developing a novel three-dimensional (3D) cell model to better mimic the in vivo microenvironment, the researchers show that KSHV induces transcriptional reprogramming of primary lymphatic endothelial cells (LEC) to mesenchymal cells via EndMT, a process implicated in promoting tumor growth and cell invasiveness. Mesenchymal markers were found co-distributed in the same cells with KSHV in primary KS tumor samples, suggesting that the 3D culture in this work succeeds in recapitulating the known heterogeneity of the cell types in KS tumors.
The results also reveal a key enzyme in cancer cell invasion, MT1-MMP, as a previously unrecognized signaling molecule downstream of Notch to induce EndMT. Moreover, the 3D KSHV-LEC transcriptome showed significant up-regulation of invasion related genes, that were found co-regulated in 3D KSHV-LECs and KS biopsies and suggesting that virus-induced EndMT may contribute to development of KS. The results further demonstrate that the 3D culture provides a permissive microenvironment for continuous viral replication and persistence, indicating the importance of virus-cell interactions for viral spread and thereby for oncogenesis.
"The unraveled molecular mechanisms can lead to identification of novel cellular targets for pharmacological control in virus-associated cancers", Dr. Ojala says.
This work is a collaboration between the research teams headed by Kaisa Lehti, Kari Alitalo, Lauri Aaltonen, and Sampsa Hautaniemi (all from University of Helsinki), Chris Boshoff (UCL Cancer Institute, University College London) and Adam Grundhoff (Heinrich Pette Institute-Leibniz Institute for Experimental Virology). The project involves scientists from two Academy of Finland National Centre of Excellence Programs, the Translational Genome-Scale Biology and Cancer Biology.