It takes a sugar to catch a sugar
After every meal, the hormone insulin is released into the bloodstream, issuing instructions to target cells to begin taking up excess sugar. In some situations, however, cells stop responding to these signals; and this insulin-resistant state is associated with onset of type 2 diabetes. Unexpected findings from Tadashi Suzuki’s group at the RIKEN Advanced Science Institute in Wako have now revealed how a cellular malfunction may contribute to this insulin resistance.
Suzuki and postdoctoral fellow Yoshimi Haga had originally sought to develop imaging strategies to track localization of proteins modified with carbohydrate groups in a process known as glycosylation. They tested their method with the glucose-transporter protein GLUT4 and developed a mutant version of the protein that lacks a glycosylation site, but the results led their study in a new direction. “We accidentally found that behavior of our N57Q mutant and the wild-type protein was quite different,” says Suzuki. “It was a completely serendipitous finding.”
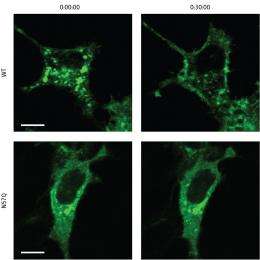
Insulin-responsive cells typically maintain reservoirs of GLUT4 in bubble-like vesicles within the cytoplasm; insulin signals induce the transport of GLUT 4 to the cell surface, where it begins pumping glucose into the cell. The N57Q mutant, on the other hand, was largely unresponsive to insulin (Fig. 1). Suzuki and colleagues determined that, instead of gathering within storage vesicles, this protein tended to accumulate either at the cell surface or within ‘recycling vesicles’ that continually shuttle to and away from the plasma membrane.
The N57Q mutant retains normal glucose-transporting capabilities, suggesting that this modification acts primarily as a trafficking signal rather than influencing protein function. Accordingly, the researchers observed the same insulin-insensitive behavior when they used a chemical treatment to alter the glycosylation of normal GLUT4. “These results clearly suggest that there must be a mechanism that detects subtle differences in glycan structure on this protein to sort it into specific GLUT4 vesicles,” says Suzuki.
Several studies have found evidence that GLUT4 may be subject to altered glycosylation in a subset of patients with type 2 diabetes; and, these new findings provide a potential explanation for how malfunctions in this protein modification process could contribute to pathology. As a next step, Suzuki hopes to uncover more details about how this transport pathway intersects with the insulin response. “We believe that glycan-recognition molecules known as lectins should be involved in this process,” he says, “and we will try to identify these lectins and other players involved in the fine-tuning of intracellular trafficking of GLUT4.”
More information: Haga, Y., Ishii, K. & Suzuki, T. N-glycosylation is critical for the stability and intracellular trafficking of GLUT4 glucose transporter. The Journal of Biological Chemistry published online 14 July 2011. doi: 10.1074/jbc.M111.253955















