Researchers attack HIV's final defenses before drug-resistant mutations emerge

Scientists who study HIV are facing a troubling consequence of their own success. They created drugs that can now give infected patients almost normal life expectancy. However, those same drugs will eventually cause the constantly mutating virus to evolve into a form that eludes current treatments.
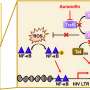
With a new $3.4 million grant from the National Institutes of Health, the University of Missouri is leading a team of researchers who want to stay a step ahead of HIV by finding new pathways for shutting down the virus. The scientists are developing new compounds designed to target an enzyme in HIV called RNase H, which has escaped the reach of existing drugs.
"Patients stay on these drugs for decades, so there will come a point where resistant strains of the virus will develop," said Stefan Sarafianos, PhD, principal investigator for the NIH project and Chancellor's Chair for Excellence in Molecular Virology at MU. "Our goal is to be ready for the mutating virus with new treatment options so we're not left empty-handed."
As HIV copies itself in humans, it can mutate into forms that escape the effects of previously effective treatments. More than 1.1 million people in the United States live with HIV infection, and 1 in 5 are unaware they are infected. HIV is one of the world's leading infectious killers, claiming more than 25 million lives over the past three decades.
"There are four enzymes present in HIV, and there are current drugs that target three of those enzymes," said Michael Parniak, PhD, co-investigator for the project and professor of microbiology and molecular genetics at the University of Pittsburgh. "RNase H is the last HIV enzyme being targeted, and there are no compounds currently in preclinical development designed for it."
Sarafianos is working with researchers at the University of Pittsburgh and University of Minnesota to design drugs that target the RNase H enzyme and inhibit its activity. While the enzyme has been extremely difficult to target so far, Sarafianos and his colleagues believe they now know enough about the structure of RNase H to launch a promising new drug development program.
Parniak has spent more than 15 years studying the RNase H enzyme and developing antiviral drugs for HIV/AIDS, and he and Sarafianos have collaborated on research projects for more than six years. Together, they solved the framework of an RNase H fragment, mapping out the molecular details of its crystal structure and providing a roadmap for their current research.
"If we know what the lock looks like, we can make the key," Sarafianos said. "Knowing the crystal structure gives us the framework for designing the most potent compounds that target the RNase H enzyme, which is a novel and key target for antiretroviral drug discovery."
Parniak, an expert in virology and pharmacological analysis, has identified more than 3,000 different leads for compounds that could target RNase H. However, he said it's Sarafianos' expertise and experience in crystal structures that will allow them to design the best compounds that will be then synthesized at the University of Minnesota.
"Dr. Sarafianos brings a fresh approach," Parniak said. "He has the perfect combination of skills and knowledge to move this project forward. If anyone can solve this, it's him."
In addition to targeting the HIV virus, the behavior present in the RNase H enzyme is also present in more common viruses, such as Hepatitis B. Two billion people worldwide have been infected with the Hepatitis B virus, and about 600,000 people die every year as a result of Hepatitis B infection.
"The knowledge that we gain for developing drugs against the RNase of HIV could eventually be applied toward designing drugs that target this enzyme in other viruses," Sarafianos said. In fact, in collaboration with John Tavis, PhD, at Saint Louis University, Sarafianos and Parniak are reporting in the journal PLoS Pathogens that one HIV RNase H inhibitor can also inhibit the Hepatitis B virus in cell culture.
Sarafianos' studies focus on unraveling the molecular details of how biomedically relevant enzymes function, how they are inhibited, how they develop drug resistance, and how those discoveries could be translated into drugs for treating human disease. He and his colleagues recently published results of a study in the journal Antimicrobial Agents and Chemotherapy demonstrating the effectiveness of a chemical compound with the potential to become a topical microbicide, which is by far more powerful than any other drug currently used for the treatment of HIV infection.
In addition to addressing HIV, Sarafianos has submitted patents for enzyme-specific inhibitors that target the Severe Acute Respiratory Syndrome (SARS) virus. In 2011, he was awarded a patent for a compound that could treat the foot-and-mouth disease virus. A member of the NIH's study section for AIDS Discovery and Development of Therapeutic Targets, Sarafianos became the University of Missouri's first Chancellor's Chair for Excellence in Molecular Virology in 2013. A member of MU's Department of Molecular Microbiology and Immunology, Sarafianos conducts his research at the university's Bond Life Sciences Center.