Researchers decipher molecular basis of bone's remarkable strength and resiliency
The bones that support our bodies are made of remarkably complex arrangements of materials—so much so that decoding the precise structure responsible for their great strength and resilience has eluded scientists' best efforts for decades.
But now, a team of researchers at MIT has finally unraveled the structure of bone with almost atom-by-atom precision, after many years of analysis by some of the world's most powerful computers and comparison with laboratory experiments to confirm the computed results. The findings, from a team led by civil engineer and materials scientist Markus Buehler, are published this week in the journal Nature Communications.
Buehler, an associate professor of civil and environmental engineering (CEE) at MIT, says the riddle was to find how two different materials—a soft, flexible biomolecule called collagen and a hard, brittle form of the mineral apatite—combine to form something that is simultaneously hard, tough and slightly flexible.
The constituents are so different that "you can't take these two materials individually and understand how bone behaves," Buehler says. Hydroxyapatite is like chalk, he says: "It's very brittle. If you try to bend it even a little, it breaks into pieces." Collagen, on the other hand, is what gelatin is made of—the very epitome of a wobbly substance.
Neither material, on its own, could provide adequate structural support for the body. "It takes the best qualities of the two substances," Buehler says. "But how this works is the big unknown."
Fitting the pieces together
The key to bone's properties, Buehler says, is in the exact way the two components fit together—which is what's been so difficult for scientists to figure out. The molecules responsible for these properties can be probed by tools such as atomic force microscopes, but only in two dimensions: on the surface, or in thin slices. But bone is an intricate three-dimensional structure, and there has been no way to image such fine structures in all their complexity.
"It's easy to get images of bone, but it's hard to see exactly where the minerals are located inside the collagen," says Shu-Wei Chang, a CEE graduate student who was a co-author of the paper.
That's where the computers come in: Buehler explains that even a few years ago, the modeling required to deduce the internal structure of bone would have taken years of computer time on the most powerful computers. But newer supercomputers can carry out much more detailed computations in a few months. Still, it took several iterations, after studying the previous results and probing the material's response to increasing pressure, to derive an answer, which the team was then able to confirm through comparisons with laboratory tests.
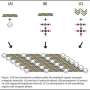
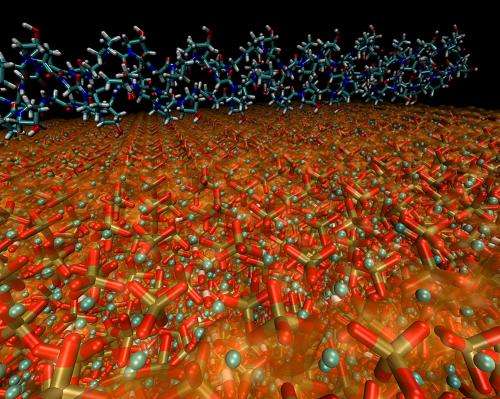
One key, they found, is that the hydroxyapatite grains are tiny, thin platelets just a few nanometers (billionths of a meter) across, and are deeply embedded in the collagen matrix. The two constituents are bound together by electrostatic interactions, which allows them to slip somewhat against each other without breaking.
The best of both
"In this arrangement of tiny hydroxyapatite grains embedded in the collagen matrix, the two materials can each contribute the best of their properties," Buehler says. "Hydroxyapatite takes most of the forces in the material, whereas collagen takes most of the stretching."
The new understanding of bone's molecular structure and function could help in unraveling what goes wrong in certain diseases, including osteoporosis and brittle bone disease. "We can use this model to understand how a bone becomes more brittle," says Arun Nair, a CEE postdoc who was the first author of the paper. For example, collagen is made up of thousands of amino acids, but "if only one of those amino acids is altered, it changes the way the minerals form" inside the bone, Nair says.
"That's why this model is so critical," he adds. Without it, you could observe how bone changes as a result of disease, but "you don't know why. Now, we can see how a very tiny change … changes the way the mineral grows, or how the forces and deformation are distributed."
Ultimately, this work could also lead to the synthesis of new bone-like materials, either as biomedical materials to substitute for bone or as new structural materials for engineering uses. "We hope we can replicate this in the lab," Buehler says.
Sandra Shefelbine, an associate professor of mechanical and industrial engineering at Northeastern University who was not involved in this research, says, "This computational molecular model … is fundamental to understanding the molecular basis for the mechanics of bone. Computational models can give us insight at length scales that are difficult to probe in experiments." The MIT team's analysis "is exciting in that it provides the molecular basis for the interaction. … This work is cutting-edge in the field of molecular modeling of structural biological tissues."
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news about MIT research, innovation and teaching.