April 26, 2013 report
Fusion and cell death in the development of skeletal muscle
(Medical Xpress)—Membrane fusion is a highly regulated event, both inside cells, and between them. From the moment a sperm first fuses with an egg, subsequent developmental events depend upon its proper control. Inside cells, fusion events regulate phagocytosis and vesicle exocytosis, as well as control proliferative and apoptotic events associated with mitochondria. Fusion between cells, as in the formation of placental trophoblasts, osteoclasts, and myoblasts, share many of the genetic and biochemical pathways used for fusion processes occurring inside cells. Developing communities of cells have also improvised, and come up with a few additional tricks of their own. In a paper just published in Nature, researchers from the University of Virginia, have taken a closer look at how myoblasts fuse in the development of skeletal muscle to become multinucleated syncytia. In particular, the researchers reveal how apoptosis in a chosen few of the myotube progenitors is critical to the process.
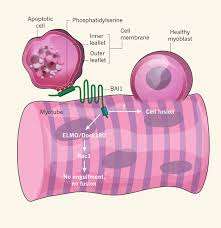
The selective localization of a critical membrane component, phosphotidylserine (PS), to the inner leaflet of cell membranes is maintained by a set of enzymes known as flippases. Some flippases require energy in the form of ATP to operate, and can transpose PS in either direction. Others operate without burning ATP, and generally flip PS from outside to inside. As such, PS is a near-universal marker that is exposed on the outer surface of cells when they undergo apoptosis. Previous studies have indicated that transient externalization of PS is required for myoblast fusion. Recognition of PS, by phagocytes, has been shown to be mediated by a membrane protein known as BAI1—a prototypical seven-transmembrane domain adhesion protein.
The Virginia researchers asked the logical question of whether BAI1 might also play a role in the fusion of myoblasts. They found a striking inhibition of myoblast fusion when PS was masked in cultures of fusing cells. To do this they used different kinds of antibodies to antagonize the binding domains involved. They next investigated whether apoptosis was required for myoblast fusion. They found that blocking the action of caspase enzymes, which prevent apoptosis, also prevented myoblast fusion.
To bolster their convictions that BAI1 is in fact transducing the PS signal in muscle development, the researchers studied its expression in the cells. They found that background BAI1 expression increased in actively fusing myotubes, and furthermore established that increasing its expression enhanced this fusion. The effect proved to be dependent on a conserved mechanism known as the ELMO/Dock/Rac1 pathway.
The researchers also generated mouse mutants lacking BAI1 to establish its role in vivo. The anterior tibialis muscle was examined in grown mice and was found to contain fully formed myotubes. However, it was observed that these myotubes contained significantly smaller fibers on average, and no large fibers at all were found. It therefore would appear that at least in the intact animal, some myotube formation can still occur, but BAI1 is required for optimal muscle development. When mature muscle was injected with cardiotoxin to induce muscle injury, mice lacking BAI1 failed to show regeneration of muscle, suggesting further important roles for BAI1.
Another interesting experiment they did was to add apoptotic myoblasts to the caspace-inhibited cultures in attempt to rescue fusion. They observed that there was a 150 percent increase in the fusion index. With that they definitively concluded that apoptotic myoblasts are necessary to promote myoblast fusion. Furthermore they noted the requirement for phosphatidylserine-dependent cell–cell contact between apoptotic and viable myoblasts.
The researchers were able to observe the fate of apoptotic myoblasts using time-lapse video microscopy. They were surprised to see that the myoblasts were not engulfed by nascent myotubes. Myoblasts did remain in close association with the myotubes and actin polymerization was observed to occur at the interface between the cells. The full story of the involvement of apoptosis in myotube generation is lacking a few details. However these new results, in addition to just introducing a few molecular players, also have shed light on some of the mechanics of this fascinating process.
More information: Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion, Nature (2013) doi:10.1038/nature12135
Abstract
Skeletal muscle arises from the fusion of precursor myoblasts into multinucleated myofibres. Although conserved transcription factors and signalling proteins involved in myogenesis have been identified, upstream regulators are less well understood. Here we report an unexpected discovery that the membrane protein BAI1, previously linked to recognition of apoptotic cells by phagocytes3, promotes myoblast fusion. Endogenous BAI1 expression increased during myoblast fusion, and BAI1 overexpression enhanced myoblast fusion by means of signalling through ELMO/Dock180/Rac1 proteins4. During myoblast fusion, a fraction of myoblasts within the population underwent apoptosis and exposed phosphatidylserine, an established ligand for BAI1 (ref. 3). Blocking apoptosis potently impaired myoblast fusion, and adding back apoptotic myoblasts restored fusion. Furthermore, primary human myoblasts could be induced to form myotubes by adding apoptotic myoblasts, even under normal growth conditions. Mechanistically, apoptotic cells did not directly fuse with the healthy myoblasts, rather the apoptotic cells induced a contact-dependent signalling with neighbours to promote fusion among the healthy myoblasts. In vivo, myofibres from Bai1−/− mice are smaller than those from wild-type littermates. Muscle regeneration after injury was also impaired in Bai1−/−mice, highlighting a role for BAI1 in mammalian myogenesis. Collectively, these data identify apoptotic cells as a new type of cue that induces signalling via the phosphatidylserine receptor BAI1 to promote fusion of healthy myoblasts, with important implications for muscle development and repair.
Commentary: Cell biology: Death brings new life to muscle, www.nature.com/nature/journal/ … ull/nature12097.html
© 2013 Medical Xpress