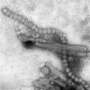
Pharma giant Novartis working on H7N9 vaccine
Swiss pharmaceuticals giant Novartis is researching a vaccine for the H7N9 strain of bird flu, its chief executive said Thursday, amid fears that the disease could mutate into a form that spreads among people.
In an interview with the Swiss daily Tagesanzeiger, Joe Jimenez said that Novartis had already analysed the virus' genetic codes, which have been published by Chinese authorities.
Jimenez said that Novartis "would today be in a position to develop a vaccine for initial clinical trials within six to eight weeks".
"The need is theoretical for now," he added.
Since the discovery of the first cases several weeks ago in China, a total of 108 people have been confirmed as being infected with the H7N9 virus, of whom 22 have died, marking the first time the strain has claimed human lives.
Officials from the World Health Organisation have underlined that the strain is therefore one of the deadliest of the many forms of flu carried by birds and posing various degrees of risk to humans.
On Wednesday, Taiwan recorded the first case outside China, in a man who had recently returned from working there.
There have been no recorded cases to human-to-human transmission, but the spectre of a form of the virus being able to jump between people has placed global health officials on alert.
(c) 2013 AFP