Booster dose of new meningitis vaccine may be beneficial
A study of 4CMenB, a new vaccine to protect against meningitis B bacteria (which can cause potentially fatal bacterial meningitis in children), shows that waning immunity induced by infant vaccination can be overcome by a booster dose at 40 months of age, according to a clinical trial published in CMAJ (Canadian Medical Association Journal).
The 4CMenB vaccine, an important breakthrough in the fight against childhood meningitis, was recently licensed in Europe and is being considered for approval in Canada and elsewhere. However, although it is known that immunizing infants with the 4CMenB vaccine induces a good immune response (as shown by an increase in antibodies against this bacteria), it was not known how well this response persists through childhood. This is critically important to the potential impact of the vaccine, because children remain at risk from this infection through their preschool years, and even into adolescence.
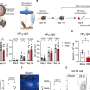
In this study of 113 children, the Oxford Vaccine Group (part of the University of Oxford Department of Paediatrics) shows that high antibody levels observed 1 month after receiving 4CMenB at 2, 4, 6 and 12 months of age decline over the following 2 ½ years. Blood samples taken from participants were tested against a panel of different strains of the meningitis B bacteria. Against some strains the antibody levels remained higher than in children who had never received the vaccine, while against other strains there was no obvious difference.
"Experience with other meningococcal vaccines has shown that waning of bactericidal antibody titres was associated with a decline in vaccine effectiveness following infant vaccination with serogroup C meningococcal conjugate vaccines," writes Dr. Matthew Snape, Department of Pediatrics, University of Oxford and National Institute for Health Research Oxford Biomedical Research Centre, Oxford, UK, with coauthors.
After receiving a booster dose at 40 months of age, 89%–100% of the children had high antibody levels for all but 1 of the 8 Meningitis B strains tested.
"Consistent with other vaccines against meningococcal disease, a waning of hSBA titres was observed after infant vaccination with 4CMenB. A booster dose during preschool years was well tolerated," write the authors.
"If 4CMenB were to be introduced into a routine vaccination schedule, measures such as adequate disease surveillance would be important to determine whether waning of antibodies might influence the effectiveness of a vaccination campaign against this bacterium," they conclude.
More information: Paper: www.cmaj.ca/lookup/doi/10.1503/cmaj.130257