Rare gene mutation sheds light on protein's role in brain development
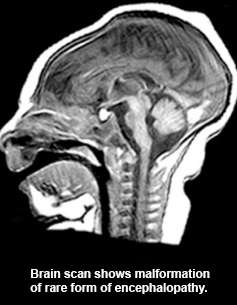
Though worlds apart, four unrelated families have been united in a medical mystery over the source of a rare inherited disorder that results in their children being born with abnormal brain growth and severe functional impairments.
An international team of scientists, led by genetic researchers at Duke Medicine, has solved the case by identifying a recessive gene mutation that reduces the abundance of a certain protein that previously had not been known to affect brain development.
The gene mutation causes a defect in the body's synthesis of a nutrient called asparagine, which is found in meat, dairy and nuts, among other foods. Long considered a "non-essential" amino acid, asparagine synthesis may actually be crucial for normal brain development and function.
The findings appear in the Oct. 16, 2013, issue of the journal Neuron.
"This non-essential amino acid has different levels inside and outside the central nervous system, and it may be that in the central nervous system, it plays a critical role," said lead author David B. Goldstein, Ph.D., director of the Center for Human Genome Variation and professor of Molecular Genetics & Microbiology and professor of biology at Duke University School of Medicine. "What is exciting about this is if we can work out how it functions, a treatment might be asparagine supplementation in the diet."
Goldstein said the work on the rare disorder was launched after two separate families in Israel, both of Iranian Jewish ancestry, had children with similar impairments – small head circumference that grows progressively worse, accompanied by profound developmental delays and seizures.
Deducting that the families' ethnic heritage might help focus the gene quest, Goldstein and colleagues looked for gene variants that were shared by the two affected children from one of the families, but were uncommon in the general population. Of 72 such variants, three were absent in the larger population.
Of those three variants, one was also present in the child of the other family from Israel. This mutation was located in the asparagine synthetase gene, or ASNS, which controls the production of the metabolite asparagine from other amino acids.
Meanwhile, two other families – both in Canada – had children who were born with similar problems, and scientists there conducted analyses that pointed to mutations in the same ASNS genes.
In combining the cases, the researchers discovered that each of the parents in these four families shared a rare recessive trait that, by chance, combined to result in a newly identified disorder in their children. More cases are likely to come to light now that the gene mutation has been identified.
Goldstein said other similar deficiencies in amino acids synthesization – all causing neurological problems - have recently been identified. These conditions have shown improvement with the use of dietary supplements, suggesting that the impairments caused by the ASNS mutation might benefit from asparagine supplementation.
"An emerging theme is that with these 'non-essential' amino acids, their metabolism does matter," Goldstein said. "This metabolic pathway is important, and it may be that the amount of asparagine is the key, or a buildup of toxin in that pathway caused by the mutation."
Goldstein said future research in mice bred to have a similar disorder could prove enlightening. Already, he said, experiments have shown that mice with ASNS mutations have a less severe form of the disorder, perhaps because they have higher levels of asparagine in their bloodstream. That insight, he said, adds hope to the prospect that dietary supplementation might diminish the impact of the mutation.
"We can now use these mice to investigate the appropriate quantities and timing of the asparagine dietary supplementation," said lead author Elizabeth Ruzzo. "Given that this is a developmental disorder it is possible that adjusting the mother's diet before she is even pregnant will be most effective."