Innovative research tool pinpoints potential therapies for multiple sclerosis

Using a novel screening platform to rapidly evaluate the cellular effects of 1,000 chemical compounds, a team led by UC San Francisco scientists has identified eight drugs that may stimulate nervous system repair in multiple sclerosis (MS).
All eight compounds have previously been approved by the U.S. Food and Drug Administration (FDA) for the treatment of other conditions. One of the most promising agents is an antihistamine, though the scientists caution that MS patients should not use the drug until clinical trials have established whether it can safely and effectively treat MS, and if it does, what the proper dosages and treatment regimens would be. Because of the drug's emergence as a clear front-runner in the new study, a Phase 2 clinical trial to evaluate its effectiveness in MS is already underway at UCSF.
"A major unmet need in the development of therapeutics for repair in MS has been the ability to screen compounds in a high-throughput manner," said Jonah Chan, PhD, the Debbie and Andy Rachleff Distinguished Professor of Neurology at UCSF and senior author of the new study. "Through a great deal of serendipity, combined with the hard work of outstanding students and colleagues, we have been able to address this need, and I am happy that we are already testing one compound in the clinic."
The new research was published online July 6, 2014 in Nature Medicine.
The decision to focus on compounds already approved by the FDA was driven by study co-author Stephen L. Hauser, MD, the Robert A. Fishman Professor and chair of the Department of Neurology at UCSF. As founder and director of UCSF's interdisciplinary MS Research Group, Hauser has championed efforts to translate insights from basic neuroscience research into new therapies as quickly as possible. The new study is an exemplar of that strategy: only 14 months have elapsed since the team performed the first drug screen, and the Phase 2 trial is already at its halfway point.
Co-author Ari Green, MD, Debbie and Andy Rachleff Distinguished Professor of Neurology, is principal investigator on the Phase 2 trial at UCSF, which is known as the ReBUILD trial. According to Green, the trial was expedited by the FDA's granting of a New Drug Application exemption, which allows clinical researchers to study drugs in conditions for which they were not originally approved. The trial is still enrolling MS patients and is expected to be completed by the end of 2014.
In MS, the immune system goes awry and attacks myelin, a fatty sheath covering the thin nerve-cell extensions called axons that transmit signals in the brain. Much like the plastic covering on electrical wiring, myelin provides insulation that is crucial to quick, efficient communication among neurons. Poor neural conduction leads to the range of progressively worsening symptoms of MS. Myelin degeneration damages axons and ultimately causes nerve cells to die off.
Myelin is formed by specialized cells called oligodendrocytes, which wrap themselves around axons in multiple layers. This wrapping process, known as myelination, has generally been studied in combined cultures of neurons and oligodendrocytes, and until recently it was widely believed that axons provide some chemical signal to oligodendrocytes that initiates myelination.
But in 2012, Chan and colleagues published studies showing that oligodendrocytes will myelinate synthetic "nanofibers" of approximately the same diameter as axons. Though this work showed that it was possible to study myelination in oligodendrocytes alone, the configuration of the fibers used in the experiments made it difficult to automate the detection and quantification of myelination, which are essential criteria to efficiently screen drugs that might stimulate remyelination to treat MS.
To address these problems, Chan's research group designed a new system based around precisely fabricated conical "micropillars." Each micropillar is only a few thousandths of an inch thick at its base, and 10,000 of them can fit within a 5-millimeter-square "well." Chan's team created plates of 96 micropillar wells and loaded up each well with 40,000 oligodendrocyte precursor cells (OPCs), the cells from which oligodendrocytes are derived in the brain and spinal cord.
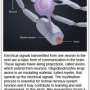
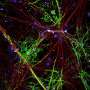
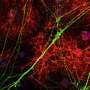
OPCs do not always differentiate into myelin-forming oligodendrocytes, so the research team tagged the cells with fluorescent markers that would glow green if the cells remained OPCs, and glow red if they had become oligodendrocytes.
The group then systematically applied 1,000 compounds from a library of FDA-approved drugs to the wells with an automated screening platform. Using a confocal microscope to view the slides from below, the researchers could quickly determine from the color of the cells if they had differentiated into oligodendrocytes, and could also calculate how thoroughly any oligodendrocytes had wrapped the micropillars—from beneath the micropillars, myelination is seen in cross-section, and quantifying it is much like counting tree rings.
In 2013, Chan was the inaugural winner of the Barancik Prize for Innovation in MS Research from the National Multiple Sclerosis Society for his work on the new platform, which is known as BIMA (Binary Indicant for Myelination Using Micropillar Arrays).
The vast majority of the compounds tested with the BIMA platform in the new study killed the OPCs or were not beneficial to their development, and many prompted the OPCs to proliferate without transforming to oligodendrocytes. But eight drugs stood out on two counts: they successfully prompted OPCs to differentiate into oligodendrocytes, and the resulting oligodendrocytes robustly wrapped the micropillars with layers of myelin.
Remarkably, all eight drugs share a common mechanism of action: they each block a particular receptor—called the muscarinic receptor—on a subset of OPCs that respond to the neurotransmitter acetylcholine.
The antihistamine clemastine was the most effective of all 1,000 compounds tested in promoting both oligodendrocyte production and myelination. The drug exerts some of its anti-allergy effects by blocking the actions of histamine in mucous membranes, but the drug also has an "off-target" effect, blocking muscarinic receptors in the brain and elsewhere in the body.
"It is imperative that we exploit and utilize the power of our screening platform to search for additional compounds, but another next step is to identify the receptor targets of these anti-muscarinic drugs so we can develop therapeutic compounds with minimal side effects," said Chan. "There are five different muscarinic receptors expressed in the nervous system, and a major question is whether the effects we observed are the result of blocking a single receptor or a combination of multiple receptors. Understanding the molecular mechanisms responsible for oligodendrocyte differentiation and myelination will provide valuable insight into the repair process and guide the development of new effective therapeutics for remyelination."
More information: Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis, Nature Medicine, www.nature.com/nm/journal/vaop … nt/full/nm.3618.html