Drug for rare blood disorder developed at Penn receives orphan drug status from EU
A Penn Medicine-developed drug has received orphan status in Europe this week for the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare, life-threatening disease that causes anemia due to destruction of red blood cells and thrombosis. Orphan status brings such benefits as tax incentives, market exclusivity for 10 years, possibilities for additional research funding, and additional guidance from the European Medicines Agency during clinical development. This designation for the compound, called AMY-101, will allow Amyndas, the company currently developing the compound, to proceed with expedited clinical development.
AMY-101 is a new way to fight PNH, which is currently only treatable with the most expensive drug available for sale in the United States. The new strategy is based on inhibiting C3, a central component of the oldest part of the human immune system – called "complement"—and could turn out to be less costly and more effective for the majority of patients with this rare blood disorder.
Complement is a network of more than 50 proteins in the blood and on cell surfaces, part of the innate immune system, that quietly cruise the body, keeping a low profile until triggered into action. But this defense system can also be inappropriately activated and attack cells, contributing to a broad spectrum of immune, inflammatory, and age-related diseases.
John Lambris, PhD, the Dr. Ralph and Sallie Weaver Professor of Research Medicine in the Department of Pathology and Laboratory Medicine in the Perelman School of Medicine, studies this early-warning system and how to correct it when its response goes overboard. Lambris developed AMY-101 at Penn and the university licensed it to Amyndas, which is now further developing the compound for application in the clinic.
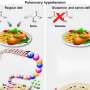
PNH affects between 1 and 5 per million people and is caused by a defective expression of regulatory proteins on the surface of blood cells, leaving them vulnerable to complement attack. This can lead to premature death of the red blood cells, a process called hemolysis, which results in severe anemia and contributes to a high risk of clotting. AMY-101 tames this inappropriate complement activation and protects cell surfaces from attack.
Although there is an expensive treatment for PNH, one third of patients continue to require blood transfusions to manage their anemia. This non-response is due to fragments of complement C3 proteins on the surface of their red blood cells, which are eventually attacked by immune cells.
Lambris and colleagues harnessed the idea that inhibition of the complement cascade using small inhibitory molecules like AMY-101 would be a better strategy to prevent hemolysis and immune cell recognition while being potentially more cost-effective.
The team investigated the effect of AMY-101 on self attack and resulting hemolysis using human PNH cells, and found it be active. The Orphan Drug designation of AMY-101 by the European Union is the next step toward clinical trials for PNH patients and orphan drug status designation from the U.S. Food and Drug Administration.