New hope in fight against muscular dystrophy
Research at Stockholm's KTH Royal Institute of Technology offers hope to those who suffer from Duchenne muscular dystrophy, an incurable, debilitating disease that cuts young lives short.
An international team that includes KTH researchers Christina Al-Khalili Szigyarto and Mathias Uhlén report that they discovered how to create a variant of dystrophin that can mitigate muscle atrophy. This could in turn lead to the development of new therapies for muscular dystrophy.
The research was published this month in Nature Medicine.
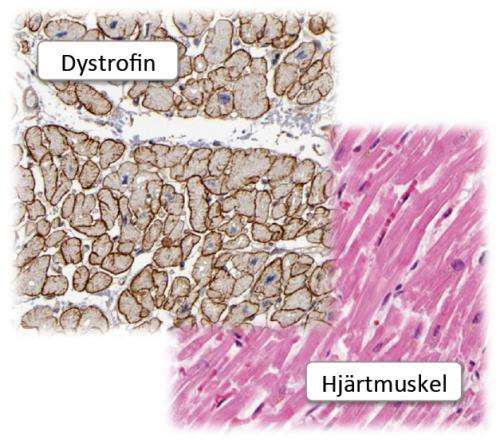
Duchenne muscular dystrophy results from a lack, or impaired function, of the protein dystrophin, a major component of muscles. Dystrophin plays a significant role in, among other things, cardiovascular functioning.
The incurable disease causes a progressive degeneration process of the muscles, resulting in decreased mobility, breathing problems, heart failure and, ultimately, a premature death.
Uhlén, a professor of microbiology at KTH, says the research team demonstrated the presence of so-called native and truncated dystrophin with the help of HPA antibodies in muscle.
"Then the protein is run through a gel, extracted and sequenced by mass spectrometry," Uhlén says. "The resulting sequences of the native and the truncated dystrophin protein have been compared on the level of amino acid.
"We have been able to demonstrate that in comparison with healthy people, the patients in the study manufacture a shorter version of the dystrophin protein despite a severe mutation in the dystrophin gene."
More information: Nicolas Wein, Adeline Vulin, Maria S Falzarano, Christina Al-Khalili Szigyarto, Baijayanta Maiti, Andrew Findlay, Kristin N Heller, Mathias Uhlén, Baskar Bakthavachalu, Messina, Giuseppe Vita, Chiara Passarelli, Francesca Gualandi, Steve Wilton, Louise R Rodino-Klapac, Lin Yang, Diane M Dunn, Daniel R Schoenberg, Robert B Weiss, Michael T Howard, Alessandra Ferlini & Kevin M Flanigan, "Translation from a DMD exon 5 IRES results in a functional dystrophin isoform that attenuates dystrophinopathy in humans and mice," Nature Medicine (2014) DOI: 10.1038/nm.3628, Received 31 January 2014 Accepted 05 June 2014 Published online 10 August 2014