Study adds diabetes drug with anti-cancer effect to ovarian cancer treatment
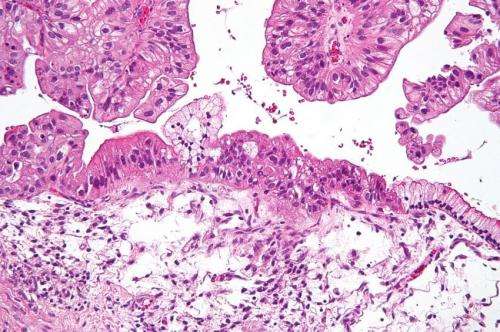
Several recent studies have suggested that metformin, an established drug developed to treat patients with type II diabetes, may provide significant benefits, including increased survival, to patients being treated for advanced cancers. An analysis of combined results from these earlier studies found that metformin use was associated with a significant decrease in cancer risk, tumor burden and cancer mortality.
The University of Chicago Medicine is leading, with two other centers, a clinical trial that will compare the most effective current therapy for patients with stage 3 or stage 4 ovarian cancer against that same therapy plus metformin. To enroll in the trial, volunteers must have a presumed or confirmed diagnosis of ovarian, fallopian tube, or primary peritoneal carcinoma, but not diabetes.
"This is the first study of its kind in ovarian cancer," said trial director Diane Yamada, MD, professor of obstetrics and gynecology at the University of Chicago. "We think this is an exciting opportunity to find out if a safe, well-tested and inexpensive drug can significantly improve on our best current therapy. There is a strong biological rationale, a series of consistently encouraging results from observational studies, and a real need for better, cost-effective therapies for this type of cancer."
Studies performed at the University of Chicago and at the Mayo Clinic have independently found that ovarian cancer patients who happened to be taking metformin for their diabetes while going through cancer treatment had significantly better outcomes. In the Chicago study, 63 percent of patients taking metformin for their diabetes were alive five years later, compared with 37 percent of patients who did not have diabetes and 23 percent of patients who had diabetes but were not taking metformin.
"More and better treatments are needed," Yamada said. Ovarian cancer has the highest fatality rate of all the gynecologic malignancies. Although more than 80 percent of patients initially respond to treatment, the majority of women with advanced stage disease will suffer a recurrence.
Laboratory studies suggest that metformin can inhibit tumor growth, alter the interaction between cancer cells and their immediate environment, and make tumor cells more sensitive to the chemotherapy used to treat advanced ovarian cancers.
Standard care for advanced ovarian cancer consists of the drugs carboplatin plus either paclitaxel or docetaxel and surgery to remove as much of the disease as possible.
In this trial, half of the patients will receive chemotherapy plus metformin, a pill, to be taken twice a day. The other half will take a placebo, a pill that looks just like metformin but contains no active ingredients. Once the chemotherapy is completed, patients in the trial will continue to take their pills, metformin or placebo, as maintenance therapy, for an extended period.
Neither the patients nor their physicians will know which subjects receive metformin and which subjects do not until the study is completed. The physicians will continue to follow patients in the study until the last subject enrolled has completed her metformin or placebo.
The medications being tested have been used extensively in ovarian cancer patients. They all have side effects. Common symptoms of carboplatin treatment may include fatigue, risk of infections and reductions in blood counts.
Paclitaxel has side effects similar to carboplatin, but can also cause allergic reactions, neuropathy and hair loss. Docetaxel's profile also includes fluid retention.
Metformin has fewer and milder side effects than these anticancer drugs, but it can cause indigestion, bloating, liver dysfunction and anemia. People with certain health problems, such as congestive heart failure or excessive alcohol use, cannot take this drug because of a higher risk for lactic acidosis. Volunteers with those problems will not be allowed to participate in this study.
The researchers hope to enroll a total of 160 patients at the three sites in the study. About 60 are expected to enroll at the University of Chicago, the primary site, and about 40 at each of the other two sites, the Evanston, IL, based North Shore University Health System, and the Mayo Clinic, based in Rochester, MN.
Study participants will not be billed for research-related expenses, such as the cost of metformin, but patients and their insurance companies will be responsible for the costs of usual medical care for ovarian cancer. This may include deductibles and co-payments.