New targeted chemotherapy technology proves effective in mice
UC San Francisco researchers have developed a new variety of targeting system for chemotherapy drugs based on the unusually high free iron content of many cancer cells—distinct from the protein-bound iron that is common throughout the body's cells. In experiments in mice and cancer cell lines, the researchers succeeded in selectively killing off cancer cells while avoiding chemo's typical toxic effects on healthy cells, enabling them to boost the tolerable dose by as much as 50-fold.
The new approach—which involves enclosing a potent chemotherapy drug within a protective chemical framework that only releases the drug when it encounters the high-iron environment of a tumor—could add a valuable new tool to oncologists' increasingly precise pharmaceutical arsenal, enabling them to target cancers with much higher doses of chemotherapy drugs while significantly reducing the grueling side effects for patients.
"Given chemotherapy's primary role in cancer treatment, we must continue to find ways to enhance its efficacy while mitigating the side effects, which can often be debilitating for patients," said Alan Ashworth, PhD, FRS, president of the UCSF Helen Diller Family Comprehensive Cancer Center. "These findings are exciting and promising because they offer clues into exploiting a tumor's vulnerabilities while thwarting drug resistance and recurrence down the line."
The new study, published in its final version online December 12, 2016 in the Journal of Medicinal Chemistry, was conducted in the lab of Adam Renslo, PhD, a UCSF associate professor of pharmaceutical chemistry and member of the Helen Diller Family Comprehensive Cancer Center, and led by then-graduate student Benjamin Spangler, PhD.
"Traditional chemotherapy drugs are very effective at killing cells, but generally they are not very selective, which is why patients experience such terrible side effects," Renslo said. "In contrast, new oncogene-targeted drugs are much more selective, but they're typically only effective against very particular forms of cancer—plus, the tumors are frequently able to evolve resistance and recur. This new approach combines some of the benefits of each: it has the inescapable destructive power of chemotherapy, but targets that power selectively to cancer cells."
New tumor-activated 'prodrug' is modeled after iron-triggered antimalarial agents
The new research grew out of Renslo's earlier work with synthetic antimalarial agents inspired by the natural product and antimalarial drug artemisinin, which selectively attacks the malarial parasite by sensing the high concentrations of free ferrous iron heme—a reactive form of iron that is rare in normal cells and tissues—in the digestive compartment of the hemoglobin-eating parasite. Exposure to this reactive free iron triggers a chemical reaction within artemisinin that leads to toxic byproducts that kill the parasite.
Renslo and colleagues have worked to create a new class of targeted prodrugs—a term for drugs which are inert when ingested but are triggered to become active by processes within the body—by designing a molecular scaffold, which they dubbed TRX, that only breaks apart and releases its active form in the presence of free ferrous iron.
Scientists have long known that malignant cancer cells similarly contain elevated levels of free iron as a result of the unnaturally revved up metabolism required for tumor cells to rapidly divide and spread. To test whether their iron-activated prodrug could target cancer as well, they adapted the TRX molecule to carry one of two different extremely potent chemotherapy drugs and tested them against a wide variety of cancer cell lines and two different mouse models of cancer.
"We chose two very potent and toxic chemicals on purpose," Renslo said. "These compounds are too toxic and non-selective to be used in patients on their own, but we wanted to show that even these drugs could be safe and effective if they could be targeted to tumors."
The authors showed that the TRX scaffold successfully blocked the toxic properties of these drugs in healthy cell cultures, but released their toxic cargo when introduced into a diverse array of cancer cell lines. Non-cancerous cells that were initially unaffected by the drug became susceptible when researchers introduced genetic mutations that drove them toward a cancerous state.
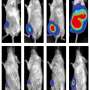
Finally, the researchers tested one of the two tumor-activated prodrugs in mouse xenograft models, in which human cancers are grafted onto laboratory mice. They established that very little of the toxic chemotherapy agent was released outside of the tumors, and, as a result, the mice could tolerate a 50-fold higher dose of the prodrug than the chemotherapy agent on its own. A combination of the higher doses and the prodrug's improved targeting to cancer cells resulted in robust and lasting reduction of the tumors in these mice.
"There's a lot to do before this approach makes its way to the clinic," Renslo added. "We're now trying to understand which are the types of cancers where this could have the greatest impact – that is, those where there aren't effective drugs available, and those that are particularly high in free iron and therefore would be likely to activate the prodrugs efficiently."
One aspect of the new approach that is particularly exciting to the researchers—though it is as yet untested—is that cancers would be less likely to evolve resistance to iron-targeted chemotherapy.
"As far as we know, malignant cancers must acquire and maintain elevated free ferrous iron in order to replicate and spread," Renslo said. "They cannot switch to another metal for these purposes and so any mutations in cancer cells that reduced their free iron enough to avoid triggering the prodrug would likely also make the cells less aggressive. We think there's nowhere they can hide."
More information: Benjamin Spangler et al. A Novel Tumor-Activated Prodrug Strategy Targeting Ferrous Iron Is Effective in Multiple Preclinical Cancer Models, Journal of Medicinal Chemistry (2016). DOI: 10.1021/acs.jmedchem.6b01470