New mRNA cancer vaccine triggers fierce immune response to fight malignant brain tumor
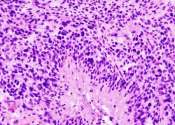
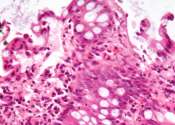
In a first-ever human clinical trial of four adult patients, an mRNA cancer vaccine developed at the University of Florida quickly reprogrammed the immune system to attack glioblastoma, the most aggressive and lethal brain ...
May 1, 2024
0
357