Researchers discover benefits of adding immune-boosting agent to personalized cancer vaccine
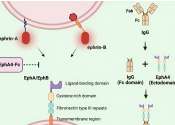
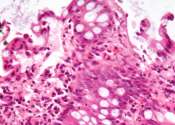
Investigators at the UCLA Health Jonsson Comprehensive Cancer Center have pinpointed a combination immunotherapy treatment that enhances the immune response for people with malignant gliomas, an aggressive type of brain tumor ...
May 9, 2024
0
18