New Details of Tuberculosis Protein-Cleaving Machinery Revealed
(PhysOrg.com) -- Scientists looking for new ways to fight tuberculosis (TB) have their sights set on a structure essential to the bacterium's survival. Disabling this structure could kill the microbes in the infected host and thwart TB infections.
In a study appearing online May 11, 2010, in EMBO J, the journal of the European Molecular Biology Organization, scientists from the U.S. Department of Energy's (DOE) Brookhaven National Laboratory, Stony Brook University (SBU), and Weill Cornell Medical College describe new features of how this structure, known as a proteasome, is put together and how it works. These details could assist researchers working to develop anti-TB drugs.
"Mycobacterium tuberculosis, the bacterium that causes TB, infects one person in three worldwide, so finding new ways to battle this pathogen is a major public health priority —particularly in developing nations where active TB infections are endemic," said study co-author Huilin Li, a Brookhaven biophysicist and associate professor at SBU.
Earlier studies by Li and his collaborators revealed important structural details of the Mycobacterium tuberculosis proteasome, a piece of cellular machinery that carves up unwanted or damaged proteins, allowing the bacterium to evade a key defense of the human immune system. The team has even identified small molecules that might be incorporated into drugs to inhibit the proteasome. "The primary aim of this new study was to look at how the proteasome, comprised of 28 proteins, is constructed," said Li.
The scientists used Brookhaven Lab's National Synchrotron Light Source (NSLS) — a source of intense x-ray, ultraviolet, and infrared light — and a cryo-electron microscope to take molecular-level snapshots of the proteasome at various stages of assembly. The studies revealed important intermediate steps and changes in the shapes of the components making up the completed structure.
The snapshots also reveal how one component in particular can inhibit the assembly process.
"Such detailed understanding of the assembly process might suggest novel approaches for developing anti-TB drugs by preventing the maturation of the proteasome," said Li. "This would be an alternative to the traditional approach of inhibiting the activity of the mature proteasome."
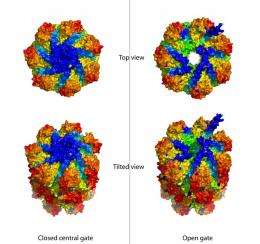
The researchers were also curious to find out how the Mycobacterium tuberculosis proteasome keeps the entrance to the protein-cleaving chamber shut.
"The fully constructed proteasome is literally a death chamber for cellular proteins, so the passage to the chamber has to be safely closed, and open only when necessary," Li explained.
Higher-level organisms, such as humans or yeast, also have gate-closed proteasomes to degrade unwanted proteins. In these cases, the gate closure mechanism is known and straightforward: Each of the seven end proteins is different, and they can assume different conformations, or shapes, to open and close the gate.
But in bacterial proteasomes, the seven end proteins are identical. "The question has been how the same protein sequence takes on the necessarily different conformations in order to seal the central pore," Li said.
Images taken by the scientists using x-ray beams at the NSLS reveal an asymmetric and tightly closed gate structure at the seven-fold symmetrical entrance. The scientists also snapped additional images showing that the gate structure retains some flexibility.
"This flexibility may be key to opening the gate to allow entry to proteins that need to be degraded," Li said. "Figuring out how to reduce the flexibility, and thus keep the gate permanently shut, could be yet another strategy in developing proteasome-targeting anti-TB drugs."
These new approaches are particularly attractive because the differences in assembly and gating mechanisms between human and TB proteasomes are more significant than the differences in the enzyme active sites that have been primary targets for drug development. As a result, drugs designed to inactivate these aspects of the TB proteasome would be less likely to also inhibit proteasomes in human cells.
TB Survival Mechanism
Most people infected with TB remain symptom-free because the bacterium is kept in check within immune system cells. These cells produce compounds such as nitric oxide, which scientists believe damage or destroy the bacteria’s proteins. If allowed to accumulate, the damaged proteins would kill the bacteria. But the TB proteasome, a protein-cleaving complex, carves up the damaged proteins, allowing Mycobacterium tuberculosis to survive, and possibly go on to cause active infections. Scientists looking for new anti-TB drugs are using the latest details about the structure and assembly of the TB proteasome to find ways to disable this action.
















