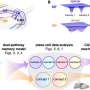
NETs catch platelets and induce clots, linking inflammation with thrombosis
(PhysOrg.com) -- Neutrophils are the innate immune system's 'first responders,' fighting infection in several distinct ways. These white blood cells can engulf foreign particles via phagocytosis, or they may release antimicrobial compounds, including granular proteins. Neutrophils are also known to sacrifice themselves, extruding their nuclear contents as a sticky antimicrobial web called a neutrophil extracellular trap, or NET.
Lead author Tobias Fuchs and others from Denisa Wagner's lab in the Program in Cellular and Molecular Medicine and the Immune Disease Institute (affiliated with both Children's Hospital Boston and Harvard Medical School) have discovered important new functions of NETs. Reporting in Proceedings of the National Academy of Sciences, they show that NETs provide physical and biochemical support for clot formation, which may help explain the epidemiological association between infection and thrombosis already identified in a range of diseases and disorders.
Dr. Arturo Zychlinsky's group in Germany discovered that activated neutrophils continue to fight infection even after their death. The nucleus dissolves and the nuclear chromatin decondenses, after which the loosened DNA, histones, and other proteins fill the cell. As the cell lyses, the nuclear materials are expelled as a NET, which binds microorganisms to stop their spread and surrounds them with antimicrobial agents, all without causing damage to host cells.
Fuchs and his colleagues began by perfusing NETs with blood. NETs acted as both a scaffold and stimulus for thrombus formation: red blood cells were recruited, and platelets adhered, became activated, and aggregated. In addition, NETs bound plasma proteins crucial for thrombus stability, including von Willebrand factor, fibronectin, and fibrinogen. The result was a red thrombus, similar to those found in veins. DNase dismantled the NETs and prevented thrombus formation, confirming that NETs were the only pro-thrombotic scaffold present.
Like DNase, heparin also destabilized the NETs, since it removes histones from the chromatin fibers that form the NETs' backbone. In fact, Wagner's team found that stimulation of platelets with purified histones was sufficient to cause aggregation.
As Dr. Fuchs explains, "In the late 1950s it was discovered that histones kill microbes. However, histones are in the nucleus; how would they naturally come in contact with microbes? Then NETs were discovered, and it turns out that their histones contribute to antimicrobial activity. Now we find that histones themselves cause platelet aggregation. These proteins, which we knew organized chromatin remodeling inside the nucleus, have completely different functions outside the cell."
Dr. Wagner, Professor of Pathology at HMS, points out that the same is true of DNA: "It performs a completely different function outside the cell. I think we will find more of that in cellular injury. We see that certain molecules wear two hats: one for clotting and the other inflammation."
Fuchs emphasizes, "It is known that inflammation contributes to deep vein thrombosis (DVT)." With collaborators at the University of Michigan, the researchers simulated DVT in a baboon model using a balloon catheter. Immunohistochemical analysis of the resulting thrombus revealed abundant extracellular DNA and histones, indicating the presence of NETs.
Historically, NETs have consistently been associated not only with infectious processes, but also with non-infectious inflammatory conditions, including preeclampsia and small-vessel vasculitis.
Besides the direct relevance of the new findings to DVTs, Dr. Wagner believes her group's work could also have applications in such diverse conditions as lupus, sickle cell disease, pathological leukocyte activation, and wound infections. "The red flag common to all these conditions is excessive cellular adhesion in blood vessels, which could be promoted by neutrophils going into the NETs suicide cycle."
As mentioned in the new PNAS paper, DNA has been detected on platelets from patients with systemic lupus erythematosus, who are prone to venous thrombosis and impaired NET degradation. In sickle cell disease, where a lethal crisis is often precipitated by infection, RBC adhesion to NETs could also play a role. Regarding pathological leukocyte activation, targeting the extracellular DNA and histones in NETs may help prevent thrombosis.
To illustrate the behavior of NETs in infected wounds, Wagner explains that the members of her lab have adopted an exotic metaphor: "If a tiger bites you with dirty teeth, not only does the infection cause an inflammatory response, but you need to close and repair the wound. NETs both contain infection and provide a matrix with which to close the wound and promote healing."
The new findings proceed logically from previous work by the Wagner lab, which focuses on the rapid response of blood cells to injury or stress with defensive or reparatory processes. The group investigates the crucial role of adhesion molecules, the regulation of their expression, and their physiological function in both health and disease.
Dr. Wagner gives a few examples of the questions still open to investigation. "Little is known about the cellular process that shuts down the neutrophil as it produces its NET; most of the work has been in vitro and on the morphological rather than the molecular level." She continues, "We really don't even know when the NETs form; six days after the DVT we find NETs in the thrombi. We will also have to figure out their role in thrombus initiation and stability, and whether they influence degradation of the fibrin bound to them. So far we know they bind and activate platelets, so they are pro-thrombotic."
However, this publication by Dr. Fuchs and colleagues demonstrates that a deeper understanding of NETs will yield applications in a broad range of diseases and disorders, as the group continues exploring this new connection between inflammation and thrombosis.
More information: Tobias A. Fuchs, Alexander Brill, Daniel Duerschmied, Daphne Schatzberg, Marc Monestier, Daniel D. Myers, Jr., Shirley K. Wrobleski, Thomas W. Wakefield, John H. Hartwig, and Denisa D. Wagner. "Extracellular DNA traps promote thrombosis". Proc. Natl. Acad. Sci. USA, E-pub Aug. 23, 2010.