Non-human primate studies reveal promising vaccine approach for HIV
(PhysOrg.com) -- Research conducted at Oregon Health & Science University's Vaccine and Gene Therapy Institute (VGTI) has developed a vaccine candidate in non-human primates that may eventually lead to a vaccine against Human Immunodeficiency Virus (HIV). Details of this advance are published in the advance online edition of the journal Nature. The paper will also be published in an upcoming print addition of the journal.
The research team, led by Louis Picker, M.D., associate director of the OHSU VGTI and director of the VGTI's vaccine program, produced a vaccine candidate that programs the immune system of non-human primates to respond more swiftly to the presence of a primate version of HIV than it normally would. The team also included researchers from the National Cancer Institute-Frederick and the International AIDS Vaccine Initiative.
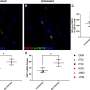
The VGTI researchers tested their vaccine candidate in rhesus macaque monkeys at the Oregon National Primate Research Center using a monkey form of HIV called Simian Immunodeficiency Virus (SIV). Of the monkeys that received the vaccine candidate, just more than half controlled replication of the virus to the extent that even the most sensitive tests could not detect signs of SIV.
To date, the vast majority of these animals have maintained control over the virus for more than a year, gradually losing any signs that they had ever been infected. In contrast, the macaques in the unvaccinated control group developed the monkey form of AIDS.
The researchers say that their work suggests that the immune responses elicited by this new vaccine candidate might completely clear SIV from animals that were initially infected. In comparison, antiretroviral therapy is able to control the disease, but cannot clear the virus from its hiding place within the immune systems own cells.
The VGTI team has been working for over ten years on its vaccine candidate, which is unique in using Cytomegalovirus (CMV) as the transport system used to introduce the vaccine into the body. CMV was chosen because it is believed that most people are already infected with CMV, but for the majority, the virus causes little or no symptoms. In addition, once a person is infected with CMV, this virus remains in the body for life. Picker and his team hypothesized that if such a persistent virus were used as a vector it could create and maintain resistance against HIV by programming a portion of the body's immune system called effector memory T-cells to be constantly on the alert for the virus.
"The next step in vaccine development is to test the vaccine candidate in clinical trials in humans. For a human vaccine the CMV vector would be weakened sufficiently so that it does not cause illness, but will still protect against HIV, " said Dr. Picker.
More information: Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine, www.nature.com/nature/journal/ … ull/nature10003.html
Abstract
The acquired immunodeficiency syndrome (AIDS)-causing lentiviruses human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) effectively evade host immunity and, once established, infections with these viruses are only rarely controlled by immunological mechanisms. However, the initial establishment of infection in the first few days after mucosal exposure, before viral dissemination and massive replication, may be more vulnerable to immune control4. Here we report that SIV vaccines that include rhesus cytomegalovirus (RhCMV) vectors5 establish indefinitely persistent, high-frequency, SIV-specific effector memory T-cell (TEM) responses at potential sites of SIV replication in rhesus macaques and stringently control highly pathogenic SIVMAC239 infection early after mucosal challenge. Thirteen of twenty-four rhesus macaques receiving either RhCMV vectors alone or RhCMV vectors followed by adenovirus 5 (Ad5) vectors (versus 0 of 9 DNA/Ad5-vaccinated rhesus macaques) manifested early complete control of SIV (undetectable plasma virus), and in twelve of these thirteen animals we observed long-term (≥1 year) protection. This was characterized by: occasional blips of plasma viraemia that ultimately waned; predominantly undetectable cell-associated viral load in blood and lymph node mononuclear cells; no depletion of effector-site CD4+ memory T cells; no induction or boosting of SIV Env-specific antibodies; and induction and then loss of T-cell responses to an SIV protein (Vif) not included in the RhCMV vectors. Protection correlated with the magnitude of the peak SIV-specific CD8+ T-cell responses in the vaccine phase, and occurred without anamnestic T-cell responses. Remarkably, long-term RhCMV vector-associated SIV control was insensitive to either CD8+ or CD4+ lymphocyte depletion and, at necropsy, cell-associated SIV was only occasionally measurable at the limit of detection with ultrasensitive assays, observations that indicate the possibility of eventual viral clearance. Thus, persistent vectors such as CMV and their associated TEM responses might significantly contribute to an efficacious HIV/AIDS vaccine.