October 27, 2011 feature
The error-correcting brain: New insights into the neurobiology of adaptive behavior
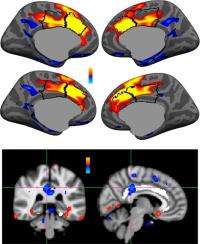
(Medical Xpress) -- A key phenomenon studied by neuroscientists is the brain’s ability to recognize errors when they occur, link them to the associated behavior, and apply those errors in a way that modifies the behavior - the overall goal being to optimize the intended result of engaging in that behavior. Two neural measurements – the error-related negativity (ERN) and error-related functional MRI (fMRI) activation of the dorsal anterior cingulate cortex (dACC, sometimes referred to as the medial frontal cortex) – have historically been seen as reflecting the same underlying neural process. Recently, however, findings by scientists at Harvard Medical School-affiliated Massachusetts General Hospital have suggested that the ERN is differentially localized to the posterior cingulate cortex (PCC).
Lead author Yigal Agam and other researchers Matti S. Hämäläinen, Adrian K. C. Lee, Kara A. Dyckman, Jesse S. Friedman, Marlisa Isom, and Nikos Makris – in the Manoach Lab run by Dara S. Manoach – faced a number of challenges in questioning the accepted view. “The error-related negativity and error-related fMRI activation of the dorsal anterior cingulate cortex have received a lot of attention as potential neural correlates of learning from errors, which is fundamental to adaptive behavior,” Agam explains. “Most theories of error processing assume that one is equivalent to the other – that they reflect the same underlying neural process – yet very few studies have attempted to directly examine this assumption. In fact, when we looked carefully at previous studies of where the ERN is coming from, although many studies found source locations that were on the fringes of, or posterior to the anterior cingulate, they assumed a source in the anterior cingulate.” This observation prompted the team to more closely examine the ERN and its relationship to fMRI activation – and doing so required collecting data from the same pool of subjects performing the same task both in the MRI scanner and during EEG and MEG recording.
Agam points out that another challenge to the interpretation of their findings was that fMRI and data from electroencephalography (EEG) and magnetoencephalography (MEG), which were compared with fMRI in the study, measure different aspects of brain activity – blood oxygenation in fMRI and electrical and magnetic fields on the scalp in EEG/MEG. The researchers therefore needed to be certain that the different localization wasn’t an artifact of the different techniques.
The team’s findings pose their own challenge to current models that view fMRI activation of the dorsal anterior cingulate cortex as the hemodynamic reflection of the ERN. “There’s debate in the literature about the various roles of the dACC and whether or not it detects errors,” Agam explains. “Much of the evidence that the dACC detects errors is based on the beliefs that the ERN is a marker of error detection and that it is generated by the dACC. While we found robust dACC activity for errors using fMRI, the EEG/MEG response to errors was earlier in the PCC and corresponded with the ERN. These findings require us to rethink the roles of these regions and how they act together to respond to, correct and learn from errors.”
One possible interpretation of their findings is that the PCC detects errors, gives rise to the ERN, and then relays error information to the dACC to implement corrective behavior. According to this formulation, dACC activation reflects a more general need to adjust behavior and exercise increased control over responses rather than error detection per se. Therefore, models need to be revised to reflect this dissociation of error-processing functions.
To achieve their results, the researchers leveraged a number of insights and innovations. “This is the first study to directly compare the ERN and dACC activation in the same group of subjects performing the same task,” Agam notes. “We don’t know if differences in previous studies were due to variability between subjects, tasks, experimental design and so on. Therefore, this question can only be addressed effectively in a multimodal imaging study like ours.”
Significantly, this study is also the first to combine EEG with MEG for localizing the generator of the ERN – an important step because EEG and MEG have different qualities: Unlike EEG, MEG signals are not distorted by the skull and scalp – but due to nature of the magnetic field, MEG only picks up electric currents that run parallel to the scalp. As a result, EEG and MEG are differentially sensitive to neural sources on the gyri and sulci (the ridges and furrows, respectively, along the folded cortical surface). Combining the two methods provides better accuracy than using either technique alone.
“Also,” Agam continues, “localizing the sources of EEG and MEG is a tricky business because you have to estimate the source based on signals recorded from the scalp.” This is called an ill-posed problem, because there is no unique solution – many different combinations of sources could generate the same measured outcome on the scalp. ”However, if you take into account our knowledge of how ERPs are generated – by pyramidal neurons oriented perpendicularly to the cortical surface – and use information from individual brain anatomy, you can vastly reduce the number of possible solutions and improve the accuracy of your estimation. To measure that anatomy, we collected high-resolution structural MRI images from each subject and used this information to estimate the location of the source in the brain.”
The researchers have their eye on ways to improve the current experimental design. “One promising technology is simultaneous recording of EEG and fMRI,” Agam notes. “Recording both signals at the same time would enable us to look at the relationship between the ERN and fMRI activation on a trial-to-trial level, allowing us to examine how variation in one error marker affects the other in a dynamic, continuous manner, as well as how they respond to the characteristics of each trial. Simultaneous recording would thereby illuminate their relationship and function.”
The group also plans to use positron emission tomography (PET) to study the involvement of dopamine, a neurotransmitter involved in error processing, in generating these markers. In addition, they’re looking at extending the current study by incorporating advanced fMRI-based connectivity analysis methods to learn how PCC and dACC act together to mediate different aspects of error processing. Moreover, Agam states, “Many research groups have done computational modeling of error processing, and our results certainly warrant renewed efforts on this front as well. A useful computational model must be true to physiological realities, and our hope is that computational models of error processing will be revised to reflect our findings concerning the different regions involved.”
A range of applications stand to benefit from the group’s findings. “Since learning from errors is critical to functioning in the world, and many neuropsychiatric disorders are characterized by rigid, repetitive behavior that is not responsive to error feedback, understanding the neural basis of error processing might illuminate the bases of these disorders and suggest pathways for intervention,” Agam observes.”For example, individuals with obsessive-compulsive disorder (OCD) show an increased ERN compared to healthy individuals. A theory advanced by Roger Pitman1 in the 1980s is that OCD is characterized by persistent and uncomfortable error signals that occur even after correct responses, and this compels behavioral repetition, or compulsions, in an ineffectual attempt to reduce the error signaling.” This idea of hyperactive and inappropriate error signaling is perfectly consistent with the clinical picture in OCD: Even though the individual may know that she or he locked the door as intended, they feel compelled to repeatedly check that it is actually locked.
Other disorders are associated with different types of abnormalities of error signaling, Agam adds. “For example, individuals with schizophrenia show a blunted ERN, and have trouble learning from errors when performing cognitive tasks. If, as we’re proposing, different functions related to learning from errors are anatomically dissociated, then functional deficits might be dissociated as well.”
In terms of next steps in their research, the team is currently analyzing their error data from three different clinical groups (schizophrenia, OCD and autism), comparing them to healthy individuals, and linking abnormalities to clinical features of these disorders. “We’re also interested in the role of dopamine in error processing,” Agam adds, “and are now studying the role of specific genetic variants that control dopaminergic function in neural error signals. Further down the line, we plan to use PET to look at activity of dopamine receptors, employ advanced analysis methods to look at interactions between different brain regions during error processing, and hopefully to use simultaneous EEG-fMRI recordings to better understand the interactions between PCC and dACC during error processing.”
More information: Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing, Published online before print October 3, 2011, PNAS October 18, 2011 vol. 108 no. 42 17556-17561, doi:10.1073/pnas.1103475108
1A cybernetic model of obsessive-compulsive psychopathology, Comprehensive Psychiatry Volume 28, Issue 4, July-August 1987, Pages 334-343, doi:10.1016/0010-440X(87)90070-8
Copyright 2011 Medical Xpress.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of Medical Xpress.



















