Results of the BRIDGE trial reported at TCT 2011
Data from the BRIDGE clinical trial demonstrate that intravenous use of the drug cangrelor was effective at maintaining platelet inhibition in patients on thienopyridines who required bypass surgery. Trial results were presented today at the 23rd annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, sponsored by the Cardiovascular Research Foundation.
Thienopyridines are anti-platelet medications that work to prevent blood clotting and improve circulation. Though they are among the most widely prescribed medications, their use can be complicated by the unanticipated need for surgery. Despite increased risk of thrombosis, guidelines recommend discontinuing thienopyridines 5 – 7 days prior to surgery to minimize bleeding.
In the BRIDGE trial, researchers sought to determine whether the investigational drug cangrelor could be used as a "bridge" between discontinuing thienopyridines and surgery.
BRIDGE is a prospective, randomized double-blind, placebo-controlled, multicenter trial in 210 patients with acute coronary syndrome (ACS) or treated with a coronary stent on a thienopyridine awaiting coronary artery bypass grafting (CABG) to receive either cangrelor or placebo after an initial open-label, dose-finding phase. After thienopyridines were stopped, patients were administered cangrelor or a placebo for at least 48 hours, which was then discontinued 1 – 6 hours prior to surgery.
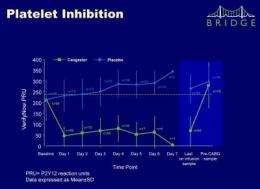
The primary efficacy endpoint of the trial was platelet reactivity (measured in P2Y12 Reaction Units [PRU]), assessed daily with the VerifyNowTM P2Y12 assay. The main safety endpoint was excessive CABG-related bleeding.
The dose of cangrelor determined in the open-label stage was 0.75 µg/kg/min. In the randomized phase, a greater proportion of patients treated with cangrelor had low levels of platelet reactivity throughout the entire treatment period compared with placebo (primary endpoint, PRU<240: 98.8% vs. 19.0%; odds ratio: 353, 95% confidence interval: 45.6-2728, p<0.001). Excessive CABG-related bleeding occurred in 11.8% vs. 10.4% in the cangrelor and placebo groups, respectively (p=0.76). There were no significant differences in major bleeding prior to CABG, although minor bleeding was numerically higher with cangrelor.
"Results of the BRIDGE trial indicate that in patients on thienopyridines who undergo cardiac surgery, intravenous cangrelor provides effective maintenance of platelet inhibition with no apparent increase in major bleeding, despite numerically higher rates of minor bleeding prior to surgery, which however were mostly attributed to ecchymosis at the site of venipuncture. Larger patient samples are needed to more definitively assert the safety and effectiveness of cangrelor bridging therapy to surgery," said Dominick J. Angiolillo, MD, PhD, the lead investigator of the trial. Dr. Angiolillo is an Associate Professor of Medicine and Director of Cardiovascular Research at the University of Florida College of Medicine in Jacksonville, Florida.















