Smallest tools could give biggest results in bone repair
When William Murphy works with some of the most powerful tools in biology, he thinks about making tools that can fit together. These constructions sound a bit like socket wrenches, which can be assembled to turn a half-inch nut in tight quarters, or to loosen a rusted-tight one-inch bolt using a very persuasive lever.
The tools used by Murphy, an associate professor of biomedical engineering and orthopedics and rehabilitation at University of Wisconsin-Madison, however, are proteins, which are vastly more flexible than socket wrenches -- and roughly 100 million times smaller. One end of his modular tool may connect to bone, while the other end may stimulate the growth of bone, blood vessels or cartilage.
On February 4th and 6th, at the Orthopedic Research Society meeting in San Francisco, Darilis Suarez-Gonzalez and Jae Sung Lee of the Murphy lab are reporting that orthopedic implants "dip-coated" with modular growth factors can stimulate bone and blood vessel growth in sheep.
For many years, medical scientists have been fascinated by growth factors -- proteins that can stimulate tissues to grow. But these factors can be too effective or not specific enough, leading to cancer rather than the controlled growth needed for healing.
Murphy wants to start applying the manifold benefits of the modular approach to healing or regenerating bone, tendon, and ligaments, and in particular to replacement surgery after an artificial joint has loosened or failed. Temporarily stimulating bones to grow by placing growth factors near the new implant could shorten healing time and ensure a good, tight fit.
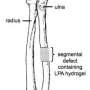
The approach could also be used for reattaching ligaments to bone after sports injuries and healing large bone defects during spinal fusion, facial reconstruction or trauma. In this work, Murphy collaborates with two associate professors of orthopedics and rehabilitation at the School of Medicine and Public Health. "Ben Graf focuses on knee injuries in sports medicine," he says, "and David Goodspeed, a lieutenant colonel in the Army who has seen blast injuries during multiple tours in Iraq, is working on the kind of major traumatic wound we think is potentially treatable using this approach."
The working end of the modular structure may feature a fragment of a growth factor, but not the entire protein. "Often, you just want the specific regions that activate the signaling pathways, because that can reduce the chances of stimulating unwanted growth, even cancer," he says.
At the other end, Murphy may place an anchoring molecule that binds to the bone and prevents the modular structure from migrating away from the wound.
With the modular approach, he says, "you might be able to stimulate bone formation without the side effects. We are trying to decrease stimulation outside of the bone defect, trying to design these molecules to specifically generate new bone in a defect, and to stay there."
Animal tests, performed in collaboration with Mark Markel, a professor of veterinary medicine, have shown that the bone is denser around the implant, and that the union between the implant and the bone is stronger than produced by state-of-the-art orthopedic techniques. The added growth factors have not been detected elsewhere in the animal, Murphy says.
Engineering each section of the molecule separately allows their properties to be tailored as needed. "We can take similar protein structures and modulate them," Murphy says. "If we want a molecule that binds very strongly to the surface of a bone graft, we can do that. If we want one that releases over controllable time-frames, we can do that as well."
Moving from the lab to the clinic is a major step, and Murphy knows that many hurdles remain. "We have shown that this can work in a large, clinically relevant animal model, but realistically, I don't see this being used in the clinic within the next five years."
Murphy says his approach is inspired by biology without trying to exactly duplicate normal communication between cells and tissues. "We are not interested in specifically mimicking a particular structure or function, but nature uses a variety of fundamental mechanisms during development and regeneration, and we are taking lessons from them and designing synthetic systems to achieve similar outcomes. We are not repeating nature, but we are inspired by nature."