First US case since FDA-approval, new magnetic device for heartburn
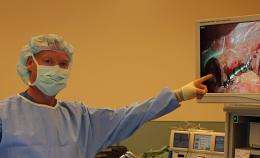
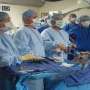
On Monday, April 9, 2012, Santiago Horgan, MD, chief of minimally invasive surgery at UC San Diego Health System implanted the new FDA-approved LINX device in a 29-year old patient suffering from gastroesophageal reflux disease (GERD), a chronic digestive disease that can lead to severe inflammation, stricture, Barrett’s esophagus and esophageal cancer.
“The multi-center clinical trial results clearly showed that the magnetic device is highly effective in treating GERD and the painful burning that results from this progressive condition,” said Horgan, an international expert in treating esophageal disease. “Unlike drugs that suppress stomach acids, this flexible device corrects the anatomy and immediately addresses the actual source of reflux.”
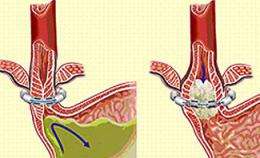
The LINX system is composed of a series of titanium beads, each with a magnetic core, that are connected to form a ring shape. It is implanted at the lower esophageal sphincter (LES), a circular band of muscle that closes the last few centimeters of the esophagus and prevents the backward flow of stomach contents.
“This device has changed my life,” said Gina Brickell, one of the first recipients of the LINX device. “I suffered from GERD for years. Now I can eat what I want, when I want, and where I want.”
The FDA approved the LINX Reflux Management System in March 2012. Horgan and his surgical team have implanted more than 20 of the devices during the clinical trial phase, representing the most surgeries in the second phase of procedures that led to FDA approval.
The LINX device is an option for patients who do not respond to dietary and lifestyle measures. The device can be placed during a 20-30 minute minimally invasive surgery. Patients may leave the hospital the same day after brief observation.
Magnetic Resonance Imaging (MRI) tests are prohibited if you have received the LINX Reflux Management System.
The LINX Reflux Management System is manufactured by Torax Medical Inc. in St. Paul, Minn.
More information: To learn more about eligibility for the LINX device at UC San Diego Health System, please call 619-471-0447.