Suit: Firm provided tainted meds in 2002, man died
The compounding pharmacy suspected in a deadly meningitis outbreak settled a lawsuit alleging it produced a tainted shot that caused a man's death in 2004, while a pharmaceutical firm with common owners was accused this summer of failing to separate sterile and non-sterile supplies.
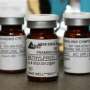
Officials have identified Framingham, Massachusetts, based-New England Compounding Center as the source of steroid shots suspected in the outbreak of rare fungal meningitis that has killed at least 12 people and made more than 130 others sick.
Allegations of a shot tainted with a different form of meningitis were at the heart of a lawsuit filed against the company over the 2004 death. An 83-year-old man died about a year and a half after receiving a shot produced by the company. And another drug company that has some of the same owners, Ameridose LLC, was accused by a business customer this year of failing to separate sterile and non-sterile products in its warehouse.
Ameridose, based in Westborough, Massachusetts, agreed to temporarily stop its compounding and manufacturing operations as a precaution while regulators inspect its facilities, but the measure is being done as a precaution, not because of evidence of contamination, officials said Wednesday.
Andrew Paven, a spokesman for both companies, said: "Ameridose is a separate entity from New England Compounding Center, with distinct operational management."
"We have separate production facilities, separate processes and operate at separate locations in different cities. Although there is common ownership, the two companies operate under separate registrations and different licensure," the statement from Paven said.
A 2004 lawsuit filed in upstate New York's Monroe County claimed that New England Compounding Center produced the shot that infected William Koch with bacterial meningitis at Rochester General Hospital on July 17, 2002. Koch died Feb. 28, 2004, at the age of 83.
The lawsuit complaint said the shot was the source of Koch's meningitis, but did not explain how that determination was made.
Bacterial meningitis is contagious and much more common than the fungal meningitis involved in the current outbreak. Fungal meningitis is more difficult to catch, according to the Centers for Disease Control.
The compounding pharmacy reached a settlement with Koch's widow in 2007 before the case went to trial, according to her lawyer Mark S. Nunn. He declined to elaborate Wednesday because the terms were confidential.
"Really all I can say is that the case settled prior to trial," Nunn said.
Two of the people who founded New England Compounding Pharmacy Inc. in 1998—Gregory Conigliaro and Barry Cadden—formed Ameridose in 2006, according to documents filed with the Massachusetts Secretary of State's office. The company's website says it provides hospitals around the country with products including intravenous solutions and prefilled oral syringes of painkillers and other medications.
This summer, an organization that represents hospitals in purchasing deals with drug suppliers cancelled a contract with Ameridose over allegations that it had poor quality control practices that "rose to a level of concern for patient safety," according to a lawsuit that Ameridose filed in August.
Ameridose denies those allegations and filed a defamation and slander lawsuit in U.S. District Court in Massachusetts on Aug. 8, saying Novation LLC hurt its reputation by making allegations including that there was "no separation between sterile and non-sterile products" in an Ameridose warehouse.
The lawsuit doesn't say what the products were or elaborate on how they were stored. Novation declined to release a copy of its report.
Novation, which leverages hospitals' combined buying power to get better prices on medical goods, sent two employees to audit Ameridose on July 15 and terminated its contract, the lawsuit said.
"Novation has determined that Ameridose does not meet the quality systems requirements needed to maintain a Novation agreement," Novation said to its members in an Aug. 2 newsletter, according to the lawsuit.
Ameridose strongly objected to the allegations and said in its defamation lawsuit that the Novation auditors were unqualified and made false and misleading statements. Ameridose also said it had been audited in recent years by several other organizations that determined its quality control system "meets or exceeds their high quality standards." Ameridose is regulated by the U.S. Food and Drug Administration.
The lawsuit ended in a confidential settlement Sept. 24.
Paven, the Ameridose and New England Compounding Center spokesman, said Wednesday in his email that the "suit involved contractual commercial issues between the companies that have since been resolved."
A statement from Novation said that while it "vigorously disputed each and every claim made in the lawsuit, the parties ultimately agreed to settle the lawsuit."
Copyright 2012 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.