Scientists build 'mechanically active' DNA material
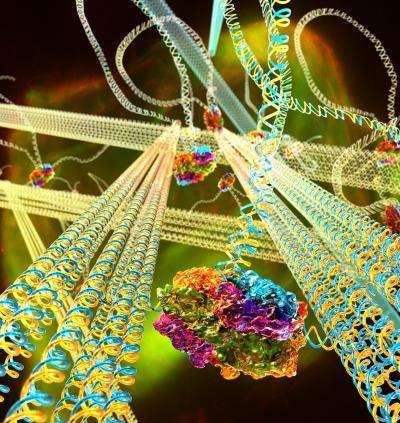
Artificial muscles and self-propelled goo may be the stuff of Hollywood fiction, but for UC Santa Barbara scientists Omar Saleh and Deborah Fygenson, the reality of it is not that far away. By blending their areas of expertise, the pair have created a dynamic gel made of DNA that mechanically responds to stimuli in much the same way that cells do. The results of their research were published online in the Proceedings of the National Academy of Sciences.
"This is a whole new kind of responsive gel, or what some might call a 'smart' material," said Saleh, associate professor of materials, affiliated with UCSB's Biomolecular Science and Engineering program. "The gel has active mechanical capabilities in that it generates forces independently, leading to changes in elasticity or shape, when fed ATP molecules for energy—much like a living cell."
Their DNA gel, at only 10 microns in width, is roughly the size of a eukaryotic cell, the type of cell of which humans are made. The miniscule gel contains within it stiff DNA nanotubes linked together by longer, flexible DNA strands that serve as the substrate for molecular motors.
"DNA gives you a lot more design control," said Fygenson, associate professor of physics and also affiliated with UCSB's BMSE program. "This system is exciting because we can build nano-scale filaments to specifications." Using DNA design, she said, they can control the stiffness of the nanotubes and the manner and extent of their cross-linking, which will determine how the gel responds to stimuli.
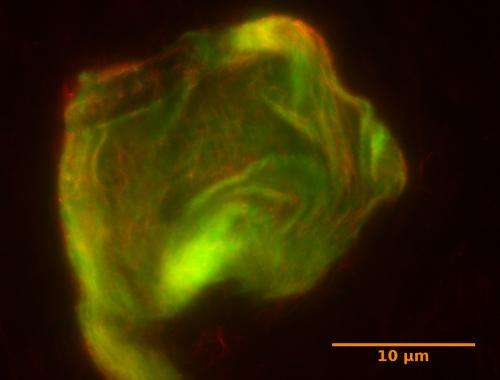
Using a bacterial motor protein called FtsK50C, the scientists can cause the gel to react in the same way cytoskeletons react to the motor protein myosin—by contracting and stiffening. The protein binds to predetermined surfaces on the long linking filaments, and reels them in, shortening them and bringing the stiffer nanotubes closer together. To determine the gel's movement the scientists attached a tiny bead to its surface and measured its position before and after activation with the motor protein.
The breakthrough, said Saleh, is that this gel "quantitatively shows similar active fluctuations and mechanics to cells."
"This new material could provide a means for controllably testing active gel mechanics in a way that will tell us more about how the cytoskeleton works," Saleh said. Like a cell, which consumes adenosine triphosphate (ATP) for energy, the DNA gel's movement runs on ATP, allowing for faster, stronger mechanics than other smart gels based on synthetic polymers.
"The development of active gels represents a water-shed event for the broader materials community," commented Craig Hawker, director of the Materials Research Laboratory at UCSB: an NSF MRSEC, which provided seed money for their research. "By exploiting cellular building blocks, it offers unique design parameters when compared to existing gel systems that can be used in a wide range of both established biomedical applications as well as totally new applications."
The project has potential applications for a variety of fields, including smart materials, artificial muscle, understanding cytoskeletal mechanics and research into nonequilibrium physics, as well as DNA nanotechnology. Long-term implications of this research are significant, Hawker added, with the final result being "a fundamental breakthrough in soft-materials science and engineering."
Having created a gel that can replicate contractions, Saleh and Fygenson are now looking to refine their technique and enable distinct movements, such as twisting and crawling, or using other motor proteins that would allow the gel to mimic other cell behaviors, such as shape-shifting and dividing.
"Biology provides a wide range of motors that we have only begun to explore," Saleh said.
"And the suite of nanostructure designs and geometries at our disposal is nearly limitless," echoed Fygenson.




















